We’ve all seen patients who present to the clinic say, “I have an eye infection.” After muttering something under our breath to the effect of “Thank you for the diagnosis, now let’s see what’s really going on,” we find that the eye is red, and the patient has variable amounts of light sensitivity, irritation and discharge. It’s not difficult to turn on the slit-lamp and see that something is clearly wrong, and occasionally we’ll have the benefit of a pathognomonic finding, such as a dendrite, to make our job easy.


Wessely immune ring: An infiltrate
associated most typically with herpetic and fungal infections. In this case, there was a bacterial source.
Migrating stromal white blood cells, seen as an area of granularity on the edge of retro beam. (This is a response to the infiltrate seen in the photo at left.)
Unfortunately, what we see more often are non-specific findings, such as injection, minimal mucous discharge, papillae or follicles (or both) and some degree of corneal infiltration, running the gamut from diffuse stromal white blood cells to a dense focal area of infiltration either with or without an overlying epithelial defect. When faced with non-specific findings, it is up to us to come to a reasonable diagnosis and form an appropriate treatment plan.
Most cases of keratoconjunctivitis can be placed into one of five subcategories (in order of frequency encountered): mechanical or dryness, inflammatory, viral infection, common bacterial pathogen and uncommon pathogen (atypical bacteria, fungus, protozoan, etc). The scope of this article covers the presentation of infectious bacterial and viral keratitides.
Barriers to Infection
The function of the cornea can be distilled into two categories: optical and structural. Optical function is predicated on the cornea’s clarity and smooth regular curvature. The structural function of the cornea is like all surface tissue, a barrier between self and non-self. The body has evolved adaptations to ensure continued function of both aspects; however, if a threat is perceived, barrier function is maintained often at the expense of optical function.
The ocular surface has a rich host of non-specific anatomic barriers (such as the lid blink reflex) and biochemical barriers (such as the presence of lysozyme in the tear film) by which infection of the cornea is minimized. When these barriers fail and infection develops, the end result—even after successful medical therapy—may involve some amount tissue contraction and scarring, either of which can limit the refractive potential of the cornea. Because of the dramatic impact infection and subsequent inflammation has on corneal tissue function, prompt and effective treatment is much more important than with a conjunctival or lid infection.

Infectious crystalline
keratitis—there are two “snowflake-like” infiltrates. The yellow in the
photo is a massive hypopyon that required surgical evacuation after the
infection had cleared (used with permission of Christopher Croasdale,
M.D.)
In addition to the generalized differences between cornea and other barrier tissue, there are also more specific differences along regions of the cornea that play a role in the immune response to infection. The cornea can be thought of as two distinct immunologic regions—the limbal/peripheral zone and the central zone.
- Limbal/peripheral zone. The peripheral cornea is near the limbal vasculature and its host of circulating immune components as well as being adjacent to the conjunctiva and its rich reserve of immune cells and lymph tissue. Additionally, there are freely distributed antigen-presenting cells in the peripheral corneal zone. Proximity to the immune response ensures a fairly vigorous response to even subtle insults. Scarring to the peripheral zone has no direct impact on paraxial light and, from a refractive standpoint, changes in peripheral cornea have less impact on the spectacle refraction the farther they are from corneal center. So, a more pronounced and rapid immune response is still able to ensure the structural function of the cornea, with little to no impact on the optical function.
- Central zone. In the central cornea, however, where changes to both clarity and curvature have a dramatic effect on paraxial optics, this is not the case. There is remoteness from any vascular tissue and immune cells are sparsely distributed.1 The disadvantage to the separation of central cornea from immunity is that there is a time lag between infection of the central cornea and an appropriately vigorous immune response. The advantage of this anatomic arrangement is singular yet key to the function of the cornea; it ensures that only true threats to the cornea activate an immune response that could cause scarring and loss of central corneal clarity.
The location-dependent duality of corneal immunity is at the source of some fairly common differences between lesions of the periphery and lesions of the paracentral zone. Sterile contact lens-related ulcers, for example, are much more common in the far periphery. The weakly antigenic agent is largely overlooked by the scant immunity of the central cornea, but generates a painful infiltrate when identified by the more active peripheral immune cells.
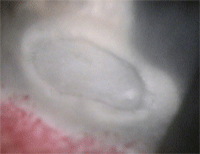
Infectious marginal
ulceration—the size of the lesion, along with the presence of an
epithelial defect, help differentiate this from a non-infectious source.
It’s also the reason limbal dendrites exist at much lower levels than their central or paracentral counterparts; the adjacent immune system more effectively contains peripheral viral activity and the characteristic dendritic appearance never develops—or more accurately, is obscured by the infiltrate generated.2
Also, keep in mind that the corneal immune response is important in determining urgency of the clinical response; however, it is less useful in differentiating pathology, which fortunately often is clear enough based on findings.
Bacterial Infection
A hallmark of bacterial cornea infection is the stromal infiltrate. While most clinically encountered infiltrates are sterile, those associated with bacterial ulceration are comprised of bacteria, necrotic and liquefied stromal tissue and white cells.
The density of the infiltrate is variable and may appear well defined, suppurative, soft or crystalline. Stromal white blood cells outside of the infiltrate are often present in increasing numbers the closer one moves towards the area of infection and give the cornea a powdery appearance.
Typically, an epithelial defect is present directly over the infiltrate and is often slightly smaller or similar in size to the infiltrate. While the presence of an epithelial defect is not mandatory in the diagnosis of a bacterial corneal ulcer, and many severe corneal ulcers begin without an epithelial defect, the lack of one is a positive prognostic sign. A typical quality of corneal healing is that, in order for stromal healing to take place, an intact epithelium is required.
While I’ve yet to hear of a practitioner misdiagnosing a bacterial corneal ulcer as viral or a dendrite as bacterial, the stromal keratitides generated by various infectious and inflammatory events do seem to create much uncertainty. Subepithelial infiltrates (SEIs) seem to be the source of greatest confusion among practitioners. This problem is encountered most frequently in contact lens wearers, but similar pathways are in play for epidemic keratoconjunctivitis (EKC), Thygeson’s superficial punctate keratitis, herpetic nummular stromal keratitis, and even corneal graft rejection manifesting as SEIs.
While the exact pathway that leads to the presence of SEIs has not been clearly elucidated in all etiologies, the clinical picture of a red photophobic eye with small focal SEIs either numbering few or multiple across the cornea in the absence of a large ulcer (either stromal or epithelial), represents an inflammatory event. Though it’s true the instigating antigens may be either bacterial or viral in nature, SEIs do not represent an actual infectious process. In most cases, the infiltrates are an accumulation of white cells presumably in the absence of actively replicating bacterial or viral particles.
The treatment in contact lens-induced cases is as simple as discontinuing lens wear for a period of time; however, SEIs of any etiology will respond very well to topical corticosteroids. A note of caution: herpetic nummular SEIs, like any herpetic immune stromal keratitis, should be treated judiciously with a “drop for drop” of prophylactic antiviral. In other words, for every drop of corticosteroid placed in the eye to treat the inflammatory event, a drop of antiviral should be used as well to prevent reactivation of any live virus still within the cornea.
Factors that must be assessed and documented when following presumed bacterial ulcers include: horizontal and vertical dimensions of the infiltrate and epithelial defect, depth of the ulcer bed, characteristics of the infiltrate margin and anterior chamber reaction. Tracking these details of ulceration will allow the clinician to quickly and accurately assess treatment success or failure and respond accordingly.
Subepithelial Infiltrates
Specific bacterial infections have known characteristic appearances that can be useful in making clinical assessments, but should not be considered diagnostic. The most frequently encountered gram-negative source of bacterial corneal infection, Pseudomonas aeruginosa—the most common source of contact lens-related microbial keratitis—causes a rapidly progressing suppurative corneal ulcer that can result in perforation.3 The appearance of this ulcer is typically wet looking with obvious necrosis of stromal tissue. Non-hemolytic streptococcal ulcers also cause a wet appearing suppurative infiltrate.
On the polar opposite side of the indolence spectrum would be certain non-tuberculous Mycobacterium species, which present as slowly developing focal dense infiltrates and low-grade inflammation, or the alpha-hemolytic Streptococci, which are the most frequent (though not only) source of infectious crystalline keratitis (ICK).4 ICK can be caused by a number of pathogenic species and presents with a radially-spoked, snowflake-like infiltrate. The development of this very defined, but subtle infiltrate is predicated on bacteria that draw a minimal stromal infiltration of white blood cells, which allows the infiltrate to progress slowly along the interlamellar spaces of collagen fibers.
Occupying the middle ground between the more aggressive infections and the more indolent infections are those caused by staphylococcal pathogens. These are characterized by a round or oval dense infiltrate with distinct margins that generate a moderate anterior segment reaction.4
Bacterial Treatment
Location of a corneal infiltrate is a key to determining the appropriate treatment strategy. A central or paracentral corneal infiltrate larger than 2mm with a pre-existing epithelial defect should be gram stained, cultured and treated aggressively (or referred for these services).
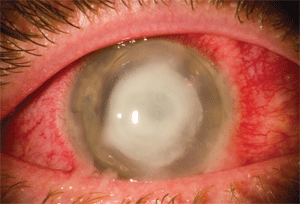
|
| Pseudomonas aeruginosa corneal ulceration (used with permission of Christopher Croasdale, M.D.)
|
A peripheral ulcer can more often be treated empirically with commercially-available prescriptions and requires culturing only if treatment proves ineffective. Proximity to immunity with peripheral ulcerations and the remoteness from corneal center often results in complete resolution with little impact on visual function.
Remember that most marginal ulcers are inflammatory in nature rather than an actively infectious process.6 While it’s only a rule of thumb, a good way to differentiate infectious peripheral ulcers from sterile ones is to assess the size and density of the infiltrate as well as presence of an epithelial defect. Larger infiltrates with large epithelial ulcerations are more consistently attributable to infectious causes.6,7 Combination therapy, such as Zylet (loteprednol/tobramycin, Bausch + Lomb) or Tobradex (tobramycin/dexamethasone, Alcon), may be used in unclear marginal cases and often proves effective.
Viral Infections
Overt infectious viral keratitis exists in limited forms, which are fairly well documented. The most easily recognized manifestation of infectious viral keratitis is the herpes simplex dendrite.
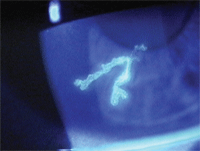
Herpetic dendrite—easily recognized as a branching epithelial ulceration with swollen terminal end bulbs.
Encountered clinically by all optometrists, the dendrite, in its classic form, is a branching epithelial ulceration with swollen terminal end bulbs. Surrounding epithelium is grey and is the site of active viral proliferation characteristic of a true infection of corneal tissue.
On detailed examination, the dendrite is ulcerated to the level of the anterior stroma, and although the stroma typically remains minimally impacted by viral activity, the subsequent inflammatory response can create an underlying infiltrate. They stain positively with fluorescein along the extent of the epithelial defect, and negatively around the margins of the defect.
While a dendrite is difficult to confuse with anything else, the pre-dendritic stage of infectious herpetic keratitis is often overlooked or vague enough to create clinical confusion.
So-called “disciform keratitis” is another inflammatory manifestation of herpetic eye disease that gives some clinicians pause. Disciform keratitis is a term used to describe a central circular area of corneal edema that arises in response to herpetic eye disease (both VZV and HSV may cause this). The clinical picture is of rapidly arising stromal edema with variable keratic precipitates and a subsequent reduction in vision. Certainly any stromal edema that presents rapidly without an etiology should raise suspicion of herpetic eye disease. The pathogenesis is presumed to be inflammatory (not infectious) endotheliitis from retained viral antigens and, as one would expect from an inflammatory condition, it is quite responsive to topical corticosteroids. In cases of disciform keratitis, oral antivirals (rather than topical antivirals due to poor tissue penetrance) may be used as prophylaxis to eliminate any worry of underlying infectious herpetic iridocyclitis.
Prior to ulceration of the epithelial layer, the lesion is vesicular in nature. These are true vesicles in the epithelial layer, which means they are elevated and will cause negative staining with fluorescein. They are usually quite clearly demarcated. Within one to two days the vesicles coalesce, the epithelium breaks down and a true dendrite is formed.
Disciform Keratitis
Presence of epithelial vesicles in an irritated eye without a history of recent trauma should immediately alert the clinician to the possibility of a herpetic etiology. Differential diagnosis of focal vesicular epithelial lesions includes epithelial basement membrane (EBMD) dystrophy and healing recurrent corneal erosions. These can be sorted through generally quite well based on history—EBMD lesions are asymptomatic while corneal erosion symptoms arise dramatically, most often upon waking. Herpes simplex virus (HSV) vesicles typically present with the concern of a gradually worsening foreign body sensation on a slightly red eye occurring over a day’s time.
Dendritic ulcers in the absence of appropriate treatment may progress to geographic ulcerations, which represent the late stage in the acute natural history of the disease. These ulcerations, while not as clearly diagnostic as a well-formed dendrite, maintain the same scalloped and raised borders that aid in their identification.
Viral Treatment
Regardless of the stage herpetic corneal ulcers present at, treatment is the same: topical antivirals. Historically, topical antivirals have been in the form of trifluridine; but more recently, topical ganciclovir (Zirgan, Bausch + Lomb) has become available.
The concomitant use of oral antivirals as an additive therapy is not directly supported by the literature, but its use remains widespread and is reasonable.8 Combining mechanical debridement of the epithelial edge of dendrites with antiviral therapy also seems to improve outcomes without negatively impacting healing rate.2,8
‘Other’ HSV Viral Ulcers
Less well-defined manifestations of infectious herpetic keratitis are the marginal ulcer and necrotizing stromal keratitis.
Marginal ulcer. The HSV marginal ulcer represents a peripheral form of dendritic keratitis. Proximity to the limbal immune response results in an infiltrate that masks the dendritic appearance and can easily be confused with a staphylococcal marginal ulcer seen in blepharitis.
Pre-dendritic vesicular HSV keratitis. These defects will not stain with sodium fluorescein.
Inappropriate treatment with corticosteroids will clarify any clinical confusion because Staph-related marginal ulcers will clear, but HSV-related marginal ulcers will get worse, move centrally and take on the more typical dendritic pattern.- Necrotizing stromal keratitis. This is a rare form of HSV-related disease that is caused by viral invasion of the corneal stroma (a feature not present in the dendritic form of the disease) and presents as a stromal infiltrate with an overlying epithelial ulceration. In other words, it looks very similar to bacterial corneal infection. When the differentiation can be made, treatment is similar for other cases of infectious herpetic keratitis.2
The presentation of bacterial corneal ulcerations and infectious viral keratitis is generally fairly dramatic and clear. Key findings in the differentiation of keratitides are the presence, size and location of an infiltrate, depth of the infiltrate, and the presence of an epithelial defect.
As corneal infections can often result in reduced vision, and occasionally loss of an eye, timely treatment is imperative.
In the grey zones, where etiology isn’t as obvious or when the magnitude of findings surpass our comfort level as clinicians, an appropriate referral constitutes good therapy.
Dr. Bronner is in practice at Hollingshead Eye Center, a secondary care center in Boise, Idaho.
1. Hattori T, Chauhan SK, Lee H, et al. Characterization of langerin-expressing dendritic cell subsets in the normal cornea. Invest Ophthalmol Vis Sci. 2011 Jun 28;52(7):4598-604.
2. Holland E, Brilakis H, Schwartz G. Herpes Simplex Keratitis. In: Krachmer JH, Mannis MJ, Holland EJ, eds. Cornea. 2nd ed. St. Louis: Mosby-Year Book; 2004:1043-74.
3. Green M, Apel A, Stapelton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27:22-27
4. Huang A, Wichiesen P, Yang M. Bacterial Keratitis. In: Krachmer JH, Mannis MJ, Holland EJ (eds). Cornea. 2nd ed. St. Louis: Mosby-Year Book; 2004:1005-29.
5. Keay L, Edwards K, Naduvilath T, et al. Microbial keratitis: Predisposing factors and morbidity. Ophthalmology. 2006 Jan;113(1):109-16.
6. Baum J, Dabezies OH Jr. Pathogenesis and treatment of “sterile” midperipheral corneal infiltrates associated with soft contact lens use. Cornea. 2000 Nov;19(6):777-81.
7. Efron N, Morgan PB. Can subtypes of contact lens-associated corneal infiltrative events be clinically differentiated? Cornea. 2006 Jun;25(5):540-4.
8. Wilhemus KR. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst Rev. 2010 Dec 8;(12):CD002898.
| A Guide to Corneal Infiltrates
| |||
|
|
Bacterial
|
Viral
|
Inflammatory
|
| Infiltrate
|
Large (greater than 1mm in size), generally dense with stromal ulceration. Infiltrate causes stromal degradation and thinning. May progress to perforation.
|
Most often, these represent an inflammatory sequelae to the actual infection rather than a true stromal infection. Often appears as a stromal infiltrate that roughly mirrors the overlying dendritic bed.
|
Marginal infiltrates are generally 1mm or smaller in the periphery or mid-periphery.* Sometimes they cannot be distinguished clearly from infectious infiltrates.
* Subepithelial infiltrates are pinpoint (or just larger) in size and may be single or multiple. |
| Epithelial
defect |
Usually present and roughly the same size as the underlying infiltrate. Not mandatory for diagnosis of bacterial keratitis, though. A general rule is that a large infiltrate with a large overlying epithelial defect is probably infectious.
|
Dendritic ulceration is the hallmark finding of infectious herpetic keratitis. Less common are marginal ulcerations. With herpes zoster ophthalmicus, corneal mucous plaques and pseudodendrites (not true ulcerations) are signs of infectious viral epitheliopathy.
|
If present, they are small in size. May actually just be a confluence of superficial punctate keratitis rather than a true epithelial defect.
|
| Corneal
edema |
Often, the cornea surrounding the infiltrate is edematous.
|
Present in cases of disciform stromal edema (which occurs without an overlying epithelial defect). This is not thought to be caused by active viral infection, though. Keratic precipitates are typically present.
|
Typically absent.
|
| Anterior
chamber reaction |
May be extensive or mild. Spectrum can include cell, flare and hypopyon.
|
HSV and HZO may both cause iridocyclitis, though these occur independently of corneal infection. May be paired with corneal stromal edema and/or elevated IOP.
|
Typically not present. In cases with an anterior chamber reaction, it is usually mild.
|

