 |
An 85-year-old Hispanic female presented for evaluation of longstanding poor vision OU. She denied any pain, redness, photophobia, photopsias or floaters. Her past medical history included hypertension, diabetes mellitus, hypothyroid and cardiac arrhythmias, all of which were controlled medically. Her past ocular history included macular hole repair OS 15 years prior with subsequent cataract extraction.
Best-corrected visual acuity was 20/50 OD and the 20/200 “E” at two feet OS. Intraocular pressure was 14mm Hg OU, confrontation fields were grossly full OU, extraocular motilities were full OU and pupils were equal in size and reactivity without a relative afferent pupillary defect. There was 2+ nuclear sclerotic cataract OD and a well-centered posterior chamber intraocular lens with open posterior capsule OS.
Take the Retina Quiz
1. Fundus imaging (Figures 1 and 2) depicts:
a. A type I posterior staphyloma OD.
b. Type III peripapillary staphyloma OS.
c. Central chorioretinal atrophy OU.
d. All of the above.
2. Which of the following is not true of the OCT of the right eye?
a. There is a retinoschisis affecting the outer plexiform layer.
b. There is an epiretinal membrane (ERM) inducing traction.
c. There is a posterior staphyloma.
d. There is peripapillary atrophy.
3. What is the most likely diagnosis for the patient’s right eye?
a. Cystoid macular edema.
b. Lamellar macular hole.
c. Myopic foveoschisis.
d. X-linked juvenile retinoschisis.
4. What is the indicated management for this patient’s right eye?
a. Observation.
b. Intravitreal anti-VEGF.
c. Macular buckle.
d. Pars plana vitrectomy with inner limiting membrane (PPV/ILM) peel and silicone oil tamponade.
5. Which of the following is not a risk factor for developing the condition in the right eye?
a. A large, wide and deep posterior staphyloma.
b. Axial length of 30mm.
c. Presence of dome-shaped macula.
d. All of the above.
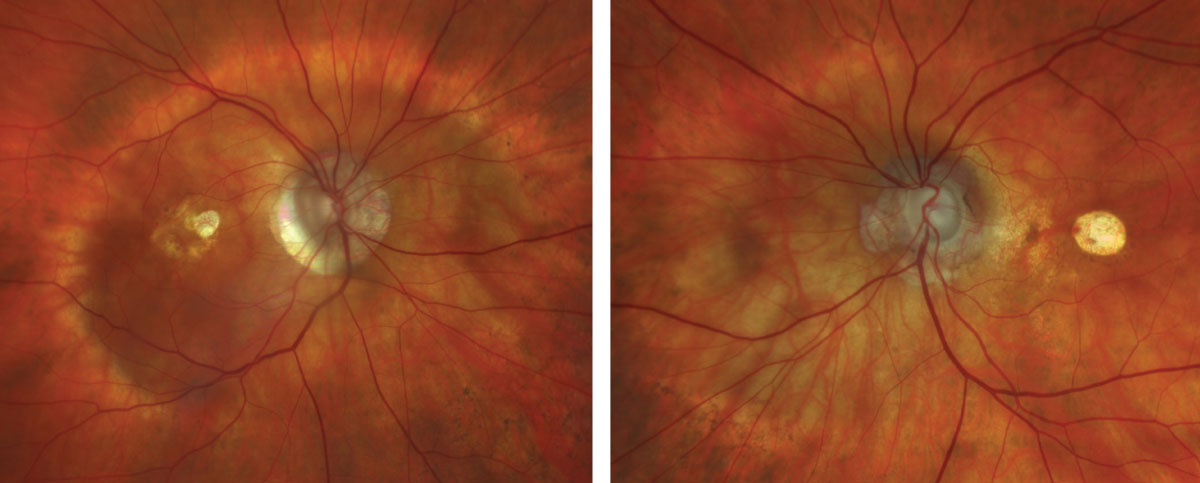 |
Fig. 1 and 2. Zeiss Clarus fundus photograph of the right eye (left) and left eye (right). Click image to enlarge. |
Diagnosis
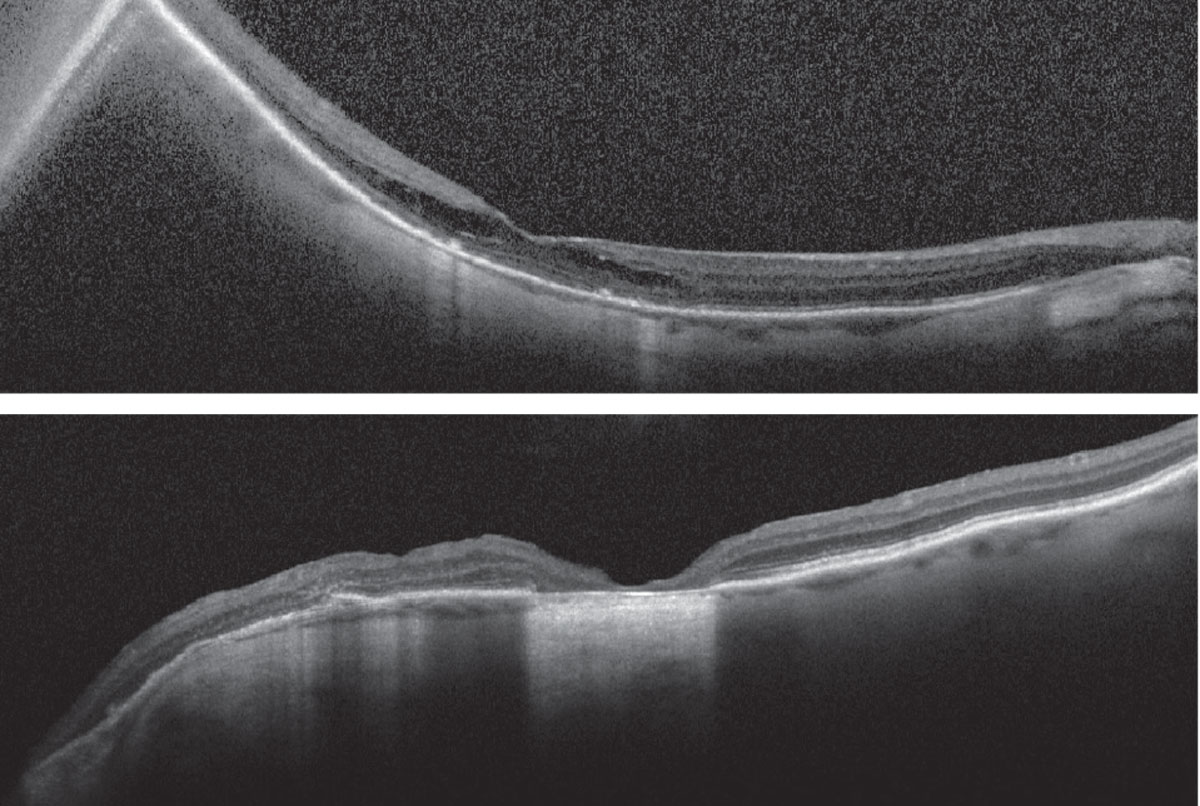
Fundus examination revealed a type I posterior staphyloma OD and type III peripapillary staphyloma OS, optic nerve cupping with peripapillary atrophy OU, central chorioretinal atrophy OS>OD and a thickened and mottled appearance to the macula OD (Figures 1 and 2). OCT revealed staphylomatous contour of the macula OD and optic nerve OS, macular thickening with outer retinoschisis OD and central retinal pigment epithelial (RPE) atrophy with nasal outer-retinal disruption and loss of typical macular architecture OS (Figures 3 and 4). It is important to acknowledge the absence of macular hole and that the retina is flat. The patient was diagnosed with myopic foveoschisis OD and central RPE atrophy involving the fovea OS following macular hole repair.
Discussion
Myopic foveoschisis is when highly myopic patients develop macular retinoschisis with or without an associated localized retinal detachment or secondary macular hole.1,2 When there are preretinal proliferative membranes causing traction on the retina, the term myopic traction maculopathy (MTM) can be more broadly applied.3 While initially described in 1959, the term myopic foveoschisis was first coined in 1999 and further expanded upon with the advent of OCT technology, which improved our ability to better understand and identify this disease.1,2
The prevalence of myopic foveoschisis has been reported to be approximately 0.8% of the general population, but can be as high as 32.9% of high myopes in the general population and 62% of high myopes in tertiary care settings.4 High myopia is generally regarded as an axial length greater than 26mm to 26.5mm or a spherical equivalent refractive error greater than -6D or -8D, depending on the study.2-5
Pathophysiology is thought to be largely related to variations in elasticity of the inner and outer retina, which may be accompanied by preretinal fibrous proliferations that can induce anteroposterior traction on the retinal surface, thereby exacerbating inequalities of retinal flexibility.1,2 The degree of tangential and anteroposterior traction exerted on the retina is probably related to the shape, diameter and depth of posterior staphylomas.3,4 To that end, it has been proposed that developing myopic foveoschisis may be more related to staphyloma progression.2 Interestingly, histopathologic studies have identified a disproportionate presence of collagen fibers, cellular debris and fibrous glial cells as compared with idiopathic nonmyopic macular holes1,2 Risk factors for development of myopic foveoschisis include spherical equivalent > -8D, axial length > 31mm, presence of posterior staphyloma, abnormal vitreoretinal interfaces (including ERM) and increasing age (typically over 50 years of age).1,2
Due to the subtle clinical nature of slight macular elevations within staphylomas, it is felt that OCT is the most useful diagnostic tool.1 Rapid advancements over the last few decades have allowed for high resolution structural analysis of the macula, aiding our ability to detect more subtle myopic foveoschisis or ERM/ILM detachments (i.e., macular schisis of the inner retina).1 MTM is a spectrum of disease in the setting of high myopia and often with posterior staphyloma, where patients may present with ERM, ILM detachment, myopic foveoschisis, foveal or macular detachment, lamellar or full-thickness macular hole (FTMH) and subsequent retinal detachment.1-3
 |
|
Figs. 3 and 4. Heidelberg Spectralis OCT of the right eye (top) and left eye (bottom). Click image to enlarge. |
Surgical Intervention
The natural history is evolution along the MTM spectrum, which often begins as inner or compound inner and outer macular schisis that progresses to primarily outer retinal macular schisis. Eventually, lamellar and FTMH may form, and a localized foveal detachment can progress to retinal detachment.5,6 The earlier stages of MTM with inner, outer or compound inner and outer macular schisis are often observed, though surgery can be considered based on severity and patient needs.1,5 Foveal detachment portends a high risk of macular hole formation, and surgical intervention is generally indicated within one to two months.1,5 Of patients with myopic foveoschisis, between 31% and 50% have been reported to develop FTMH alone within three years and FTMH with retinal detachment within two years, respectively.1
Goals of surgery are to release any abnormal vitreoretinal or preretinal traction and to tamponade the retina to restore the retinal architecture.5 Cases of FTMH repair in high myopes carry a worse single-surgery success rate than idiopathic nonmyopic macular holes due the greater mechanical tension of the globe wall within the staphyloma.1 Initial techniques were aimed at reinforcing the sclera of the posterior globe with donor tissue in the 1930s and subsequently the macular buckle in the 1950s.5 Once PPV was developed, it provided an intraocular approach to releasing vitreoretinal and preretinal traction and was later combined with ILM peeling.1,2,5 Long-acting tamponade (e.g., silicone oil or C3F8 gas) is preferred to promote restoration of retinal architecture and reattachment of the photoreceptors and RPE.1,5
Our patient was observed over three years with overall stability. Should she develop worsening of her schisis with foveal detachment, lamellar or full-thickness macular hole, surgical intervention may be indicated
Retina Quiz Answers
1: d, 2: b, 3: c, 4: a, 5: c
Dr. Aboumourad currently practices at Bascom Palmer Eye Institute in Miami. He has no financial disclosures.
1. Ryan SJ, Davis JL, Flynn HW, et al. Retina, Fifth ed. London; New York: Saunders/Elsevier, 2013. 2. Gohil R, Sivaprasad S, Han LT, et al. Myopic foveoschisis: a clinical review. Eye (Lond). 2015;29(5):593-601. 3. Panozzo G, Mercanti A. OCT findings in myopic traction maculopathy. Arch Ophthalmol. 2004;122(10):1455-60. 4. Cheong KX, Xu L, Ohno-Matsui K, et al. An evidence-based review of the epidemiology of myopic traction maculopathy. Surv Ophthalmol. 2022;67(6):1603-30. 5. Frisina R, Gius I, Palmieri M, et al. Myopic traction maculopathy: diagnostic and management strategies. Clin Ophthalmol. 2020;14:3699-708. 6. Parolini B, Palmieri M, Finzi A, et al. The new myopic traction maculopathy staging System. Eur J Ophthalmol. 2021;31(3):1299-312. |

