The much-anticipated Comparison of AMD Treatments Trials (CATT) Study found that Avastin (bevacizumab, Genentech) is as effective as Lucentis (ranibizumab, Genetech) for the treatment of neovascular AMD when administered on the same schedule after one year of dosing. The report was published online in the New England Journal of Medicine on May 1.
|
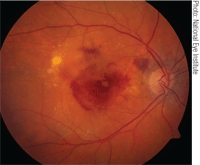
| The CATT study confirmed what many
clinicians already knew––Avastin is just as effective as Lucentis for the treatment of neovascular AMD, as seen in this patient. |
Avastin is used off-label for the treatment of AMD and is available at a lower cost than Lucentis. Lucentis is FDA approved for the treatment of AMD. Genentech originally developed Avastin to prevent blood vessel growth that enables cancerous tumors to develop and spread.
To date, the CATT researchers have reported results for 1,185 patients treated at 43 clinical centers in the U.S. Patients were randomly assigned and treated with one of four regimens for a year. They received intravitreal injects of Lucentis or Avastin either monthly or p.r.n.
Patients in the monthly dosing groups received an initial treatment and then had an injection every 28 days. Patients in the p.r.n. groups received an initial treatment and were then examined every 28 days to determine medical necessity for additional treatment. Patients in the p.r.n. groups received subsequent injections if/when they exhibited signs of disease activity, such as fluid in the retina.
Change in visual acuity served as the primary outcome measure for CATT. Thus far, visual acuity improvement was virtually identical (within one Snellen letter) for either drug when given monthly.
“The CATT study was important because it provides a scientific basis in the form of a randomized controlled clinical trial for recommending Avastin over Lucentis,” says Mark Dunbar, O.D., director of optometric services at Bascom Palmer Eye Institute in Miami. “Similarly, it will also provide insurance carriers an objective means for making a decision to pay for (or not) the more expensive drug.”
The CATT study will continue to follow patients through a second year of treatment.

