 |
A 72-year-old male presented to the ophthalmic emergency department with significantly decreased vision, pain and redness of his right eye for five days. He denied any ocular trauma. His ophthalmic surgical history was significant for cataract surgery in the left eye a number of years prior. His medical history revealed well-managed hypercholesterolemia. He was otherwise in good health and noted to be afebrile.
His visual acuity on presentation was light perception in the right eye and 20/25 in the left. His intraocular pressures (IOPs) measured at 23mm Hg OD and 12mm Hg OS. There was no view to the pupil in the right eye; the left eye’s pupil was unremarkable. The right eye had moderate diffuse conjunctival injection with turbid and opaque fluid in the anterior chamber, but the cornea was notably compact and devoid of keratic precipitates (Figures 1 and 2). There was no view posteriorly. The left eye’s exam revealed a quiet anterior chamber with a well-centered intraocular lens (IOL) and a normal-appearing posterior segment.
Due to the poor visualization inside the right eye, ultrasonography was conducted to view the ocular structures. First, a posterior segment ultrasound was completed, confirming there was no retinal detachment or mass lesion (Figure 3). Next, anterior segment ultrasonography was done, revealing a deep anterior chamber filled with dense, pin-like hyperechoic opacities. The angle was noted to be open circumferentially, and there was a thick and distended lens capsule with hyperechoic, dense lenticular material inside (Figure 4).
The patient was diagnosed with suspected phacolytic glaucoma and started on topical prednisolone acetate drops six times daily, timolol-dorzolamide drops twice daily and cyclopentolate drops twice daily in the right eye. He was asked to return the next day for surgical evaluation.
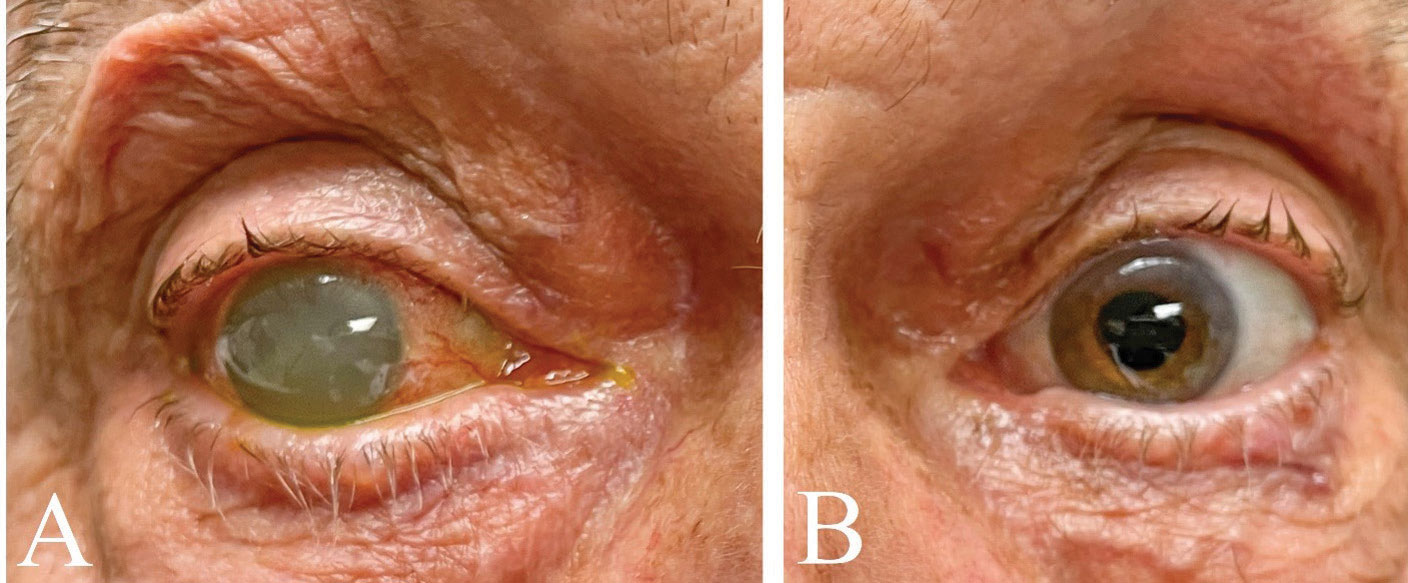 |
| Fig. 1. The right (A) and left (B) eyes of this patient on presentation. Note the cloudiness apparent in the right eye’s anterior chamber. The left eye had previously undergone cataract extraction. Click image to enlarge. |
Discussion
Somewhat of a rarity in first-world modern societies, severely advanced cataracts and their complications are not often seen. Once a cataract advances well into maturity, certain findings become more commonplace. A “white” cataract is seen clinically as a totally opaque, white lens that often precludes a view of even the retinal red reflex. A Morgagnian cataract occurs when the dense, yellowed lens nucleus drops to the bottom of the lens capsule due to liquified cortex.
Two mature lens–induced conditions that occur with an intact lens capsule include phacomorphic and phacolytic glaucoma. Although similar in nomenclature, complications and treatment, phacomorphic- and phacolytic-induced glaucoma are different entities. Both often present with severely elevated IOPs, secondary corneal edema, ocular pain and conjunctival injection. Additionally, the treatment for both is emergent management of any elevated IOP to reduce the likelihood of glaucomatous damage and urgent cataract extraction.
Phacomorphic glaucoma is characterized by a dense and thick lens pushing the iris forward and angle structures closed. The suffix -morphic refers to form or structure, and this aptly defines phacomorphic glaucoma, as the lens’s form is the primary cause for glaucomatous complications.
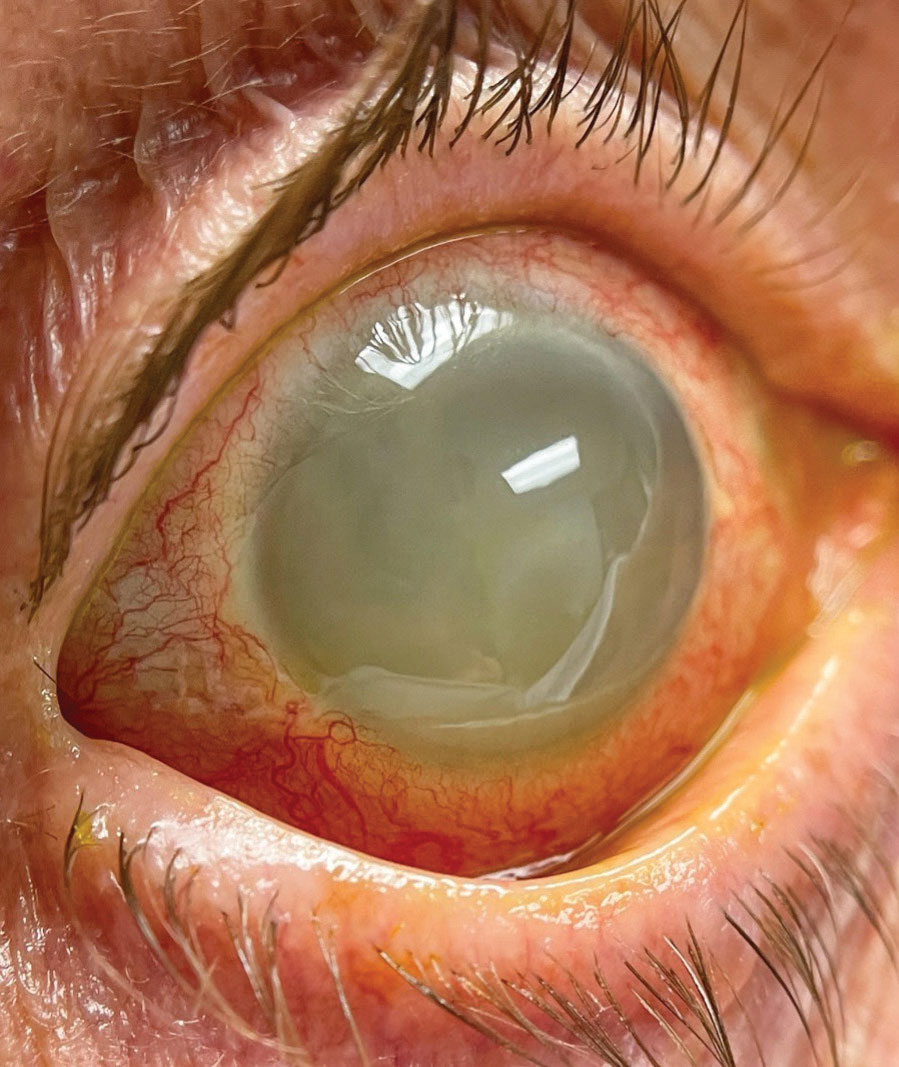 |
| Fig. 2. A magnified view of the right eye’s anterior segment. Click image to enlarge. |
Phacolytic glaucoma, on the other hand, typically has a deep chamber filled with proteinaceous lens material and macrophages. The suffix -lytic refers to lysis or decomposition of a material, and in phacolytic cases, lenticular material leaks through spontaneous micro-perforations in an otherwise intact lens capsule.
There is some thought that there may be two subtly different subtypes of phacolytic glaucoma.1 In some cases, there is a very acute onset of symptoms and IOP elevation. This is thought to likely be due to primarily liquefied lens proteins rapidly egressing through the lens capsule and into the anterior chamber, directly obstructing the angle’s trabecular meshwork. In these cases, no macrophages are seen in the anterior chamber specimen.
The other subset of cases has a slower, more gradual onset of symptoms, including the presence of macrophages with nuclear debris (ingested lens material). The outcome is the same in both cases, but the patient with hyperacute onset of symptoms will likely present earlier due to the drastic, quick rise in IOP.
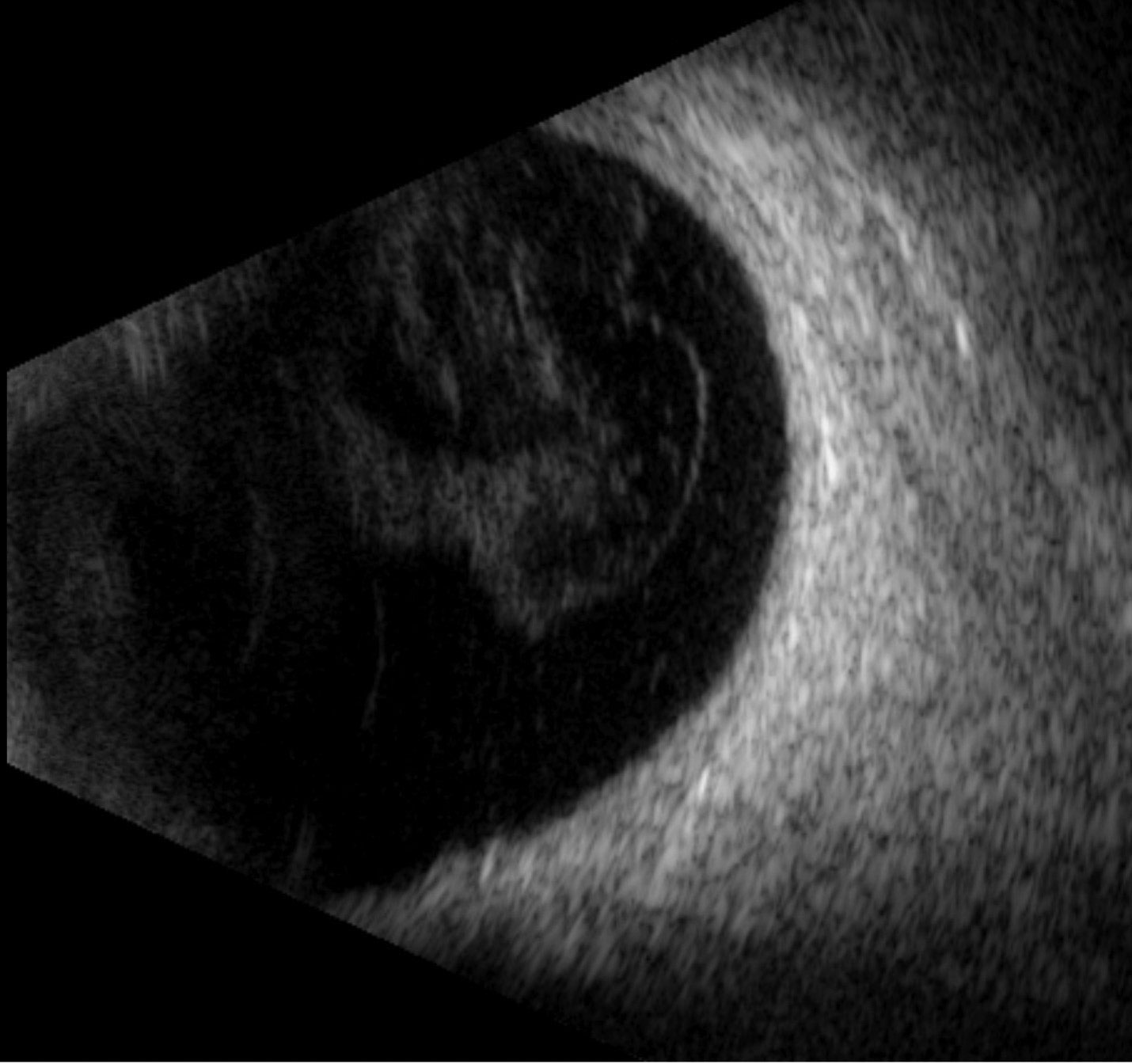 |
| Fig. 3. A normal posterior segment exam with mild vitreous opacities but no retinal detachment or lesion. Click image to enlarge. |
Interestingly, most patients who present with phacolytic glaucoma are pseudophakic in the other eye.2,3 This is likely due to the fact that they have good, functional vision in one eye and are less symptomatic for visual decline with just one advancing cataract. Therefore, doctors should educate patients and reinforce the importance of considering cataract extraction in the fellow eye before the cataract becomes hypermature.
Additionally, it is important to understand that although cataract extraction is considered curative in most cases of phacolytic glaucoma, there is still a small subset of patients who have persistent elevated IOP after surgery and require continued glaucoma management.2
Two other lens-related glaucomas that occur with lens capsule violation are lens-particle glaucoma and phacoantigenic (or phacoanaphylactic) glaucoma.
Lens-particle glaucoma occurs after macroscopic (often visible) damage to the lens capsule from trauma or iatrogenically during intraocular surgery. Once lens material is liberated into the anterior chamber, the disease mechanism leads to elevated IOP primarily due to direct particle deposit into and blockage of the trabecular meshwork.4,5
Similarly, phacoantigenic glaucoma is typically caused by surgery or trauma with capsule violation and exhibits a sensitization period of one to 14 days, during which there is a Type III hypersensitivity reaction against the lens particles.5 Often, there is significant inflammation visible, and granulomatous keratic precipitates may also be seen.
For both of these conditions, treatment is directed at managing any inflammation with topical corticosteroids, lowering IOP with topical aqueous suppressants and considering surgery if lens material remains exposed and/or does not absorb on its own.
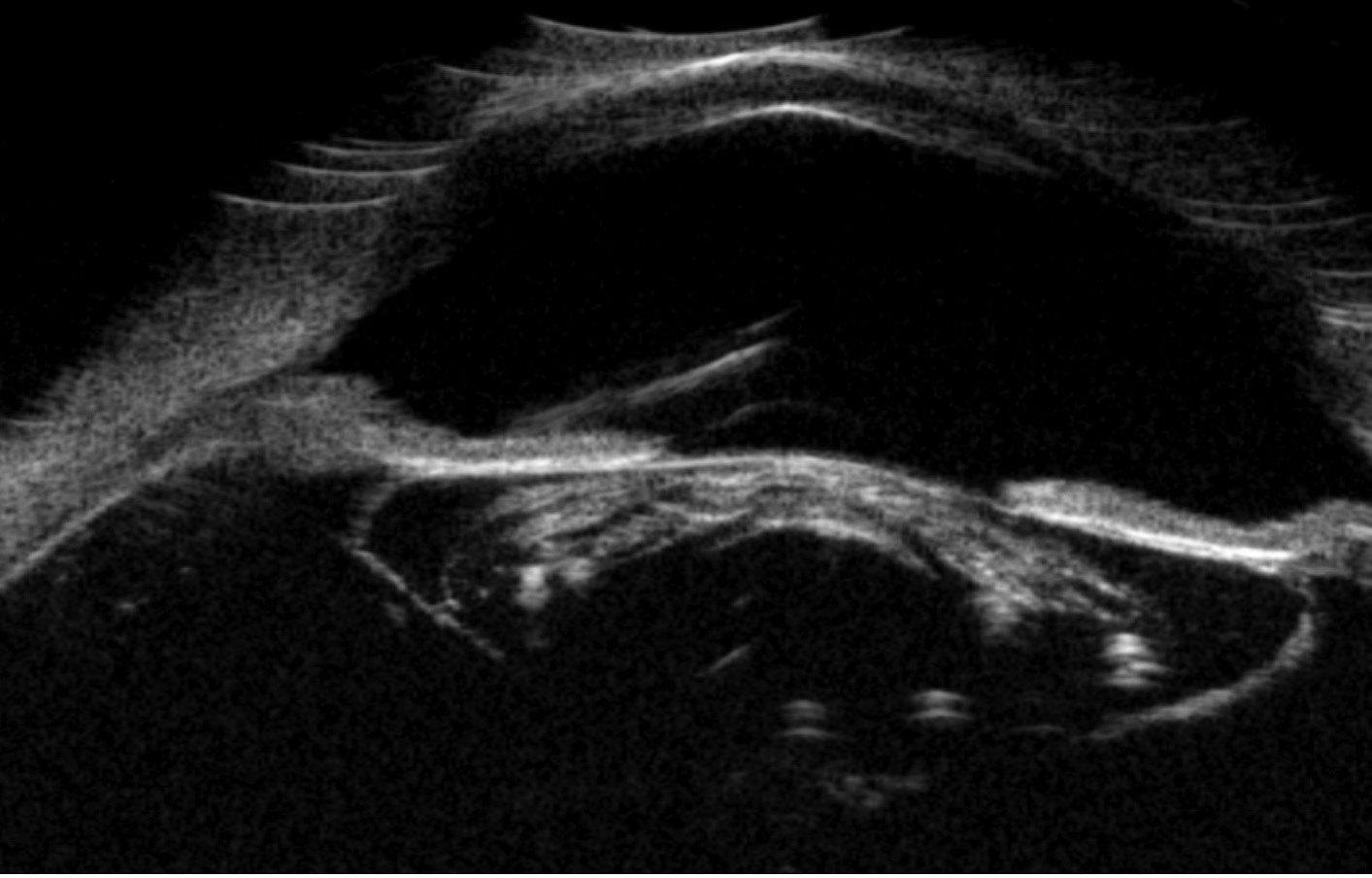 |
| Fig. 4. A thickened mature lens with a very dense cortex causing posterior shadowing. Note the anterior chamber depth is preserved. Click image to enlarge. |
Outcome
Our patient underwent cataract surgery within a few days of presentation and was noted intraoperatively to have significant zonular loss. This zonular dehiscence allowed for vitreous to prolapse anteriorly, so a pars plana vitrectomy was completed at the same time. The decision was made to remove the cataractous lens and use a sulcus-placed IOL due to poor zonular support. Mild optic atrophy was appreciable intraoperatively when the view allowed, which would likely limit the patient’s ultimate visual acuity in this eye to some extent. The pathology report of anterior chamber fluid revealed histiocytes (macrophages) with granular material within them, consistent with a subacute phacolytic process. This could be why IOP was still relatively normal on presentation compared with the high spike that is typically seen.
At the three-week follow-up, the patient’s visual acuity had improved significantly to 20/70. His IOP remained moderately elevated at 28mm Hg off IOP-lowering medication, so timolol-dorzolamide was reintroduced into his treatment plan. The patient will continue to follow-up for clinical care and glaucoma monitoring.
Dr. Bozung currently practices at Bascom Palmer where she primarily sees patients in the hospital's 24/7 ophthalmic emergency department. She also serves as the optometry residency program coordinator. Dr. Bozung is a fellow of the American Academy of Optometry and a member of the Florida and American Optometric Associations. She is a founding board member of Young OD Connect and serves on the editorial board for Review of Optometry. She has no financial interests to disclose.
|
1. Mavrakanas N, Axmann S, Van Issum C, et al. Phacolytic glaucoma: are there 2 forms? J Glaucoma. 2012;21(4):248-9. 2. Ayub R, Tom LM, Venkatesh R, Srinivasan K. Outcomes and reasons for late presentation of lens induced glaucoma: a prospective study. Ophthalmol Glaucoma. 2021;4(5):504-11. 3. Agarwal R, Bhardwaj M, Patil A, Sharma N. Phacolytic glaucoma in contralateral pseudophakes. Clin Exp Optom. 2020;103(5):708-9. 4. Dhingra D, Grover S, Kapatia G, et al. Phacolytic glaucoma: a nearly forgotten entity. Eur J Ophthalmol. 2020;30(5):NP32-5. 5. Shah SS, Meyer JJ. Lens induced glaucoma. In StatPearls. 2022: Treasure Island (FL). |

