 |
History
A 63-year-old black female presented with history of headache and decreased vision in her left eye for two weeks. Pertinent medical history included borderline diabetes managed by diet, and hyperlipidemia and hypertension controlled with medications. Her ocular history included a diagnosis of primary open-angle glaucoma suspect secondary to increased cup-to-disc ratios.
Diagnostic Data
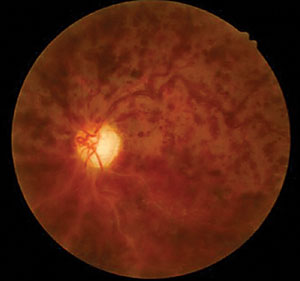
Her best-corrected acuities measured 20/20 OD and 20/60 OS. External examination was normal with no afferent defect. Peripheral confrontational fields were normal in both eyes. Central facial Amsler grid was distorted in her left eye. Biomicroscopy demonstrated normal anterior segment tissues with no evidence of pigmentary dispersion (PDS), neovascularization of the iris (NVI) or keratic precipitates (KP), in either eye. Intraocular pressures (IOP) measured 29mm Hg OD and 18mm Hg OS with Goldmann applanation tonometry. The pertinent posterior segment findings are demonstrated in the photographs.
Additional testing included gonioscopy to inspect the angle for neovascularization or additional pathology related to increased cupping and glaucoma. The test revealed open angles with no synechiae, neovascularization, exfoliation or angle recession. The angle was open to the trabecular meshwork 360 degrees around with 2+ pigment in both eyes. Additionally, photodocumentation of both nerves and fundi was completed.
A laboratory work up was suggested to the general practitioner to rule out undiscovered systemic causes for the fundus pathology and a full glaucoma workup in both eyes (perimetry, central corneal thickness and structural testing) was scheduled. Blood pressure (BP) was also measured in each arm and found to be 158/90. Additional history uncovered that the patient had self-discontinued her systemic hypertension medication for one month’s duration.
Your Diagnosis
Does this case require any additional tests? Are you able to determine a diagnosis? How would you manage this patient? What is the likely prognosis?
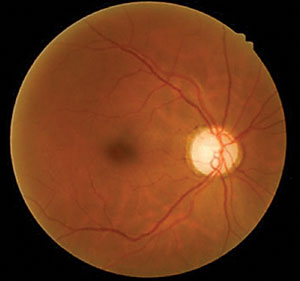 | |
| Using our 63-year-old patient’s fundus images as well as her medical history and test results, can you determine her diagnosis? | |
 |
Discussion
The diagnosis in this issue is non-ischemic central retinal vein occlusion (CRVO), OS. Ophthalmic management of CRVO should include:
- Uncovering the underlying cause through laboratory testing.
- Monitoring for anterior and posterior segment neovascularization.
- Referral to a retinal specialist to evaluate the need for vascular endothelial growth factor inhibitor (VEGF I) injection to promote fluid resolution and preservation of retinal architecture and function.
There are three classifications of retinal vein occlusion (RVO): central retinal vein occlusion (CRVO), hemiretinal vein occlusion (HRVO) and branch retinal vein occlusion (BRVO).1 With prevalence of 0.7% to 1.6%, RVO is the second most common vascular pathology behind diabetic retinopathy.2 The two most common risk factors for RVO include age and systemic vascular conditions. The prevalence of RVO increases with age.1,2 Common underlying systemic diseases include hypertension, hyperlipidemia, arteriosclerosis, and diabetes.2-4 Ocular diseases such as POAG can increase the risk of RVO.1 Other contributory factors include smoking, hyperviscosity disease, elevated fibrinogen, high plasma homocysteine, low levels of vitamin B6 and low levels of folic acid.2,3 Stroke, angina, renal dysfunction, and blood abnormalities are associated with RVO, as well as other medical conditions, including carcinoma of the breast, multiple myeloma and chronic lymphatic leukemia.2,3 The literature suggests RVOs are likely multifactorial in nature.2,3
While the pathogenesis of branch vein occlusion is largely via an arterial/venous atherosclerotic/mechanical mechanism where a hardened artery compresses a vein creating turbulent blood flow and thrombus formation. The pathogenesis of CRVO remains somewhat unclear with the mechanical element of thrombus production underplayed. The pathophysiology in CRVO includes the combination of vascular, anatomic, and inflammatory factors. Vascular pathology results from thrombus formation in the central retinal vein at or posterior to the lamina cribrosa and is often attributed to arteriosclerosis and hypertension.1,5 The occlusive event stems from apparent slowing or stasis of blood within the blood column of retinal veins which may result from increased venous pressure and decreased arterial blood flow.1 Mechanical crowding and compression of vessels within the lamina cribrosa may contribute to venous narrowing and turbulent flow, leading to vascular occlusion at the level of the lamina cribrosa.2,5 The inflammatory etiology remains under investigation.2
The first individual credited with recognizing a link between CRVO and glaucoma was Frederick H. Verhoeff, MD, in 1913. Dr. Verhoeff noted that an increase in IOP was a principle factor involved in compressing and collapsing the central retinal vein. Research today supports this relationship, as the literature suggests POAG or ocular hypertension (OHT) predispose an eye to CRVO.3,6 Loss of rim tissue associated with glaucomatous cupping causes the retinal vein to be poorly supported, and thus more readily susceptible to damage caused by fluctuations in ocular pressures.7 The central retinal vein may become kinked around the rim, leading to venous occlusion.7 The structural support provided by healthy neuroretinal rim tissue is underscored by a prevalence which finds CRVO twice as likely to develop in the setting of advanced glaucomatous cupping than in an eye free of cupping.6 Estimates of the prevalence of POAG in patients with CRVO ranges from 6% to 69%.6,8-21 Retinal vein occlusions occurring at the disc have been found to be associated with the IOP, large cups and increased prevalence of POAG more than at any other retinal site.9
Researchers studied IOP in patients with acute CRVO and HRVO, finding that prior to the occlusion, 11.4% of affected patients had elevated IOP.6 They found those with a RVO who were not being treated for ocular hypertension (OHT) had a greater likelihood of developing OHT after the occlusion.6 This study illuminated the potential for increased risk of POAG in patients with CRVO.6 Glaucoma or OHT has been found to precede CRVO in multiple studies that have examined the relationship.1,3,5
This relationship has lead thought leaders to suggest initiating ocular hypotensive therapy in order to reduce the likelihood of developing RVO in eyes with increased IOP (>24 mmHg).6 Further, those who have suffered RVO in the setting of increased cupping (>.5) should be evaluated for glaucoma.6
Although the exact IOPs at the time of RVOs has not been identified, research suggests that it must be elevated as RVO itself temporarily lowers IOP.3,5,6 The exact pathogenesis of this phenomenon remains somewhat controversial.3,5,6 Researchers have commented that a CRVO/HRVO leads to retinal hypoxia, creating a “hypotensive factor” which results in the fall of IOP post event.6
The local treatment for RVO is directed at treating three problems that can occur after the occlusion:
- Maintaining central visual acuity by minimizing macular edema.
- Treating and developing anterior and posterior segment neovascularization.
- Preventing the development of neovascular glaucoma. Additionally, it is imperative to uncover and treat the underlying systemic cause of the occlusion.
To treat macular edema, the contemporary first line therapy is VEGF I intravitreal injections, followed by intravitreal steroid therapy.22-28 There are several studies that focus on specific treatment options to optimize visual acuity, and reduce anatomic disruption.29-31
The 2012 COPERNICUS study evaluated effectivity of the newest intravitreal injection, Eylea (afibercept, Regeneron) for the treatment of macular edema.29 This study concluded that visual and anatomic improvements were observed after fixed dosing from 1-24 weeks. Further improvements diminished with as-needed dosing from weeks 24-52 and with reduced frequency monitoring from 52-100 weeks.29
The 2014 RETAIN study evaluated the long-term treatment outcome for those treated with Lucentis (ranibizumab, Genentech) for RVO.30 Of those treated for CRVO, 44% had macular edema resolution and excellent visual outcome based on letters gained and final visual acuity at 4 years, while 56% still required frequent injections and received a guarded prognosis.30
The BRAVO and CRUISE studies evaluated clinically significant visual acuity gain (15 letters or more) from baseline in patients with RVO.31 Patients were given six months of ranibizumab injections, then as-needed injections for the next six months. The results of these studies found 50% of patients treated with monthly ranibizumab achieved clinically significant visual acuity gains during the initial six months of treatment. Improvements were maintained with as-needed treatment for the next six months. Immediate treatment after diagnosis provided the greatest vision gains.31
The 2009 SCORE study found functional improvement with intravitreal corticosteroid injection versus observation alone in the treatment of CRVO.28
The Central Vein Occlusion Study evaluated the efficacy of laser photocoagulation for the treatment of macular edema and for the prevention of neovascularization.32,33 The study found macular grid-pattern photocoagulation therapy reduced the evidence of macular edema when observed angiographically, but did not improve visual acuity, and therefore was not recommended.32 The study also demonstrated that panretinal photocoagulation (PRP) for CRVO did not prevent or deter the development of anterior segment iris neovascularization or posterior segment neovascularization in eyes with 10 or more disc areas of retinal capillary nonperfusion.32,33 The study concluded prophylactic PRP was contraindicated and that anterior and posterior segment neovascularization should be treated with photocoagulation only after it is observed.33
Our Patient
This patient was diagnosed with asymmetric open angle glaucoma and prescribed latanoprost qhs OU and scheduled for a glaucoma work up. Given an absence of history of trauma and absence of pseudoexfoliation, pigment dispersion, neovascularization or uveitis, the asymmetric findings were believed to be due to the bilateral, but asymmetric nature of glaucoma. A follow up appointment was scheduled to reassess the IOP and to rule out the conversion to an ischemic CRVO with retinal or iris neovascularization two weeks after the start of the topical treatment. The patient was scheduled to see retinology within one month. The patient was referred to their internist within the week for BP evaluation and to complete the suggested work up (complete blood count with platelets and differential, lipid panel, blood sugar assessment, erythrocyte sedimentation rate (Westergren), c-reative protein, clotting analysis (prothrombin time-PT, partial thromboplastin time-PTT), sickle dex, electrocardiogram and carotid duplex testing).
The retinologist subsequently injected the patient with 1.25mg/0.05mL Avastin (bevacizumab, Genentech). Serial Heidelberg retinal tomograph (HRT) macular thickness scans were performed OS by both clinical teams. Prior to injection, macular thickness was 512µm; four weeks post-injection it was measured at 213µm. Following an additional three weeks the measurement was 376µm. At that point, persistent cystoid macular edema OS was diagnosed and the patient received an additional Avastin injection. The vein occlusion resolved over the course of seven months and the injections reduced the edema to a minimum permitting the recovery of acuity to 20/40. The patient continues to be monitored at four-month intervals for new events or worsening of her current condition.
The internist consultation was aimed at ruling out other possible etiologies of CRVO, including blood dyscrasias (hypercoagulopathy, hyperviscosity), cardiac and carotid health, hyperlipidemia, hypertension and diabetes. At the visit, her BP was measured at 108/66, however the patient admitted to self-treating with her mother’s Atenolol and Furosemide. The internist suggested inadequate monitoring of her pre-diabetic state and hypercholesterolemia to be the underlying risk factors associated with the CRVO development. Ultimately, the internist prescribed Zocor (simvastatin, Merck) and 81 mg aspirin, as well as lisinopril with an explicit note that the medication was prescribed for cardiovascular and renal health with a lesser role in treatment of hypertension.
Dr. Gurwood thanks Ashley Torreano, OD, and Julie Hutchinson McGinnis, OD, for contributing this case.
2. Ehlers JP, Fekrat S. Retinal vein occlusion: beyond the acute event. Survey of Ophthalmology. 2011;56(4):281-99.
3. Cole MD, Dodson PM, Hendeles S. Medical conditions underlying retinal vein occlusion in patients with glaucoma or ocular hypertension. Br J Ophthalmol. 1989;73(9):693-98.
4. Sherpa D, Shakya S, Shrestha JK. Association of primary glaucomas with retinal vein occlusion. Kathmandu University Medical Journal. 2008;6(1):49-54.
5. Williamson TH. Central retinal vein occlusion: what’s the story? Br J Ophthalmol. 1997; 81(8):698-704.
6. Hayreh SS, Zimmerman MB, Beri M, Podhajsky P. Intraocular pressure abnormalities associated with central and hemicentral retinal vein occlusion. J Ophthalmol. 2004;111(1):133-41.
7. Beaumont PE, Kang HK. Cup-to-disc ratio, intraocular pressure, and primary open-angle glaucoma in retinal vein occlusion. J Ophthalmol. 2002;109(2):282-6.
8. Duke-Elder WS. Text-book of Ophthalmology. Vol. III. London: Kimpton; 1936:2584–5.
9. Larsson S, Nord B. Some remarks on retinal vein thrombosis and its treatment with anticoagulants. Acta Ophthalmol(Copenh) 1950;28(2):187–201.
10. Braendstrup P. Central retinal vein thrombosis and hemorrhagic glaucoma. Acta Ophthalmol (Copenh) 1950;28(35 Suppl):1–162.
11. Becker B, Post LT. Retinal vein occlusion—Clinical and experimental observations. Am J Ophthalmol 1951;34(5):677–86.
12. Vannas S, Tarkkanen A. Retinal vein occlusion and glaucoma. Tonographic study of the incidence of glaucoma and of its prognostic significance. Br J Ophthalmol 1960;44(10):583–9.
13. Waubke T. Glaukomdisposition und Sekundärglaukom bei Thrombosen der Retinagefässe. Klin Monatsbl Augenheilkd 1960;136:224–30.
14. Bertelsen TI. The relationship between thrombosis in the retinal veins and primary glaucoma. Acta Ophthalmol (Copenh) 1961;39(4):603–13.
15. Dryden RM. Central retinal vein occlusions and chronic simple glaucoma. Arch Ophthalmol 1965;73(3):659–63.
16. Trux E. Glaucome primaire et secondaire en cas de thrombose rétinienne. Bull Mem Soc Fr Ophtalmol 1966;79:540–4.
17. Raitta C. Der Zentralvenen-und Netzhautvenenverschluss. Ein klinischer bericht über 400 fälle, unter besonderer berücksichtigung des intraocularen druckes und der späten fundusveränderungen.Acta Ophthalmol (Copenh) 1965;43(83 Suppl):1–123.
18. Vannas S, Raitta C. Intraocular hypotony vs hypertony after central retinal vein occlusion. A long-term follow-up of 95 patients. Ann Ophthalmol 1970;74(2):213–7.
19. Soni KG, Woodhouse DF. Retinal vascular occlusion as a presenting feature of glaucoma simplex. Br J Ophthalmol 1971;55(3):192–5.
20. Cappin JM, Whitelocke R. The iris in central retinal thrombosis. Proc R Soc Med 1974;67(10):1048–51.
21. Vadalà G, Zanini A, Favero C, et al. Evaluation of the clinical course of central retinal vein occlusion in eyes with and without glaucoma Acta Ophthalmol Scand 1997;75(S224):16-7.
22. Braithwaite T, Nanji AA, Greenberg PB. Anti-vascular endothelial growth factor for macular edema secondary to central retinal vein occlusion. Cochrane Database Syst Rev 2010;10:CD007325.
23. Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of phase III study. J Ophthalmol. 2010;117(6):1124-31.
24. Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. J Ophthalmol 2011;118(10):2041-49.
25. Prasad AG, Schadlu R, Apte RS. Intravitreal pharmacotherapy: applications in retinal disease. Compr Ophthalmol Update 2007; 8(5):259-269.
26. Wroblewski JJ, Wells JA 3rd, Adamis AP, et al. Pegaptanib sodium for macular edema secondary to central retinal vein occlusion. Arch Ophthalmol 2009; 127(4):374-80.
27. Brown DM, Heier JS, Clark WL, et al. Intravitreal afibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the pahse 3 COPERNICUS study. Am J Ophthalmol 2013;155(3):429-37.
28. Ip MS, Scott IU, VanVeldhuisen PC, et al. SCORE Study Research Group. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127(9):1101-14.
29.Heier JS, Clark WL, Boyer DS, et al. Intravitreal afibercept injection for macular edema due to central retinal vein occlusion: two-year results from the COPERNICUS study. J Ophthalmol. 2014;121(7):1414-20.
30. Campochiaro PA, Sophie R, Pearlman J, et al. Long-term outcomes in patients with retinal vein occlusion treated with ranibizumab: the RETAIN study. J Ophthalmol. 2014;121(1):209-19.
31. Thach AB, Yau L, Hoang C, Tuomi L. Time to clinically significant visual acuity gains after ranibizumab treatment for retinal vein occlusion: BRAVO and CRUISE trials. J Ophthalmol. 2014;121(5):1059-66.
32. The Central Vein Occlusion Study Group: Evaluation of grid photocoagulation for macular edema in central vein occlusion. The CVOS Group M Report. Ophthalmol 1995;102(10):1425-33.
33. The Central Vein Occlusion Study Group: A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The CVOS Group N Report. Ophthalmol 102(10):1424-1444.

