A 50-year-old white male was referred by his cardiologist for evaluation six days after he underwent coronary artery bypass graft surgery (CABG). The patients chief complaint: an acute onset of constant blurred vision in the superior visual field of his left eye; this had been rapidly worsening for the past week. The patient said he noticed these symptoms shortly after the surgery. He had no other complaints and denied jaw claudication, scalp tenderness, polymalgia rheumatica or headache. Differential Diagonosis
The patients ocular history was unremarkable. His medical history, however, showed coronary artery disease, carotid artery disease, hy-pertension, dysthymia, gastroeso-phageal reflux disease (GERD) and osteoarthritis. The patient also reported smoking about two packs of cigarettes per day for the last 35 years.
Leading up to the CABG surgery, the patients medical records revealed chest pain exacerbated by exertion. The pain was sharp, left sided and associated with shortness of breath, nausea, diaphoresis and radiation to both arms and neck.
These symptoms had recently increased in frequency and were now occurring during sleep. The medical records also showed that the patient underwent cardiac catheterization three days prior to surgery. The left main coronary artery and left anterior descending coronary artery were 90% blocked. The patient underwent uneventful on-pump double bypass surgery. After CABG surgery, the patient had anemia due to blood loss.
Diagnostic Data
Uncorrected visual acuity at distance measured 20/40 O.D. and finger counting at four feet O.S. Pinhole visual acuity was 20/20-2 O.D. and 20/400 O.S. Manifest refraction was +1.25D for 20/20 O.D. and +1.00D O.S. for finger counting at four feet O.S. The pupils were equal, round and reactive to light with a grade 2+ relative afferent pupillary defect O.S. Amsler grid showed no metamorphopsia or scotoma O.D. but revealed a superior scotoma O.S. Ishihara color plate testing was full O.D. and reduced to 5/16 plates O.S. Anterior segment examination was within normal limits O.U. Intraocular pressure measured 14mmHg O.U. The dilated fundus exam showed a cup-to-disc ratio of 0.20 x 0.20 O.D. The nerve was crowd-ed but had distinct margins. The left optic nerve had sectoral inferior disc edema and pallor with no hemorrhages.
The vessels were tortuous O.U. Each macula was flat with a good foveal reflex and trace hard drusen. The lens, vitreous, posterior pole and peripheral retina were unremarkable O.U.
The baseline Humphrey visual field (HVF) 24-2 SITA-fast showed scattered, non-specific defects O.D. with a borderline Glaucoma Hemifield Test. The left eye showed a dense superior altitudinal defect and an early, dense, inferior nasal step.
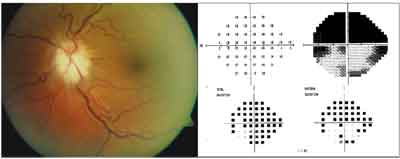
The patients left optic nerve (left) had diffuse inferior disc edema and pallor with no hemorrhages. Humphrey threshold visual field 24-2 SITA-fast showed superior altitudinal loss (right).
Diagnosis
We diagnosed the patient with non-arteritic anterior ischemic optic neuropathy (NAION) in the left eye following the CABG surgery.
Treatment and Follow-up
We instructed the patient to return in one month for follow-up evaluation. (See Differential Diagnosis, below.)
Arteritic ischemic optic neuropathy. Patients usually present with systemic symptoms of headaches, jaw claudication, scalp tenderness, neck pain and polymyalgia rheumatica. Our patient denied any of these symptoms. Also, the onset of the ION in concordance with the coronary bypass surgery enabled us to rule out any arteritic component to the ION event.
Papilledema. Presents as bilateral, diffuse optic disc swelling. This was not consistent with our patients unilateral, sectoral optic disc swelling.
Optic neuritis. Although some 33% of patients who have optic neuritis present with optic nerve swelling, it is usually accompanied by pain upon eye movement, which our patient did not have.3
Discussion
Coronary heart disease is the single largest killer of males and females in the United States. In 2002, an estimated 515,000 CABG procedures were performed on 306,000 patients.1
Anterior or posterior ischemic optic neuropathy is a rare but devastating perioperative complication from CABG surgery. Other ocular complications include retinal infarction secondary to branch and central retinal artery occlusion as sequelae of microemboli being dislocated during the CABG surgery.
NAION is a manifestation of reduced perfusion to the paraoptic branches of the short posterior ciliary arteries that supply the optic nerve as it exits the scleral canal.2 Ischemia is typically caused by atherosclerosis but can also result from hypotension, anemia and vasculitic factors. These ischemic events can initiate a vicious cycle of optic nerve swelling and nerve fiber death due to an obstruction of axoplasmic transport.
 |
| Vision loss secondary to NAION is a serious complication of coronary artery bypass graft surgery. |
As the optic nerve edema increases, the peripapillary retina is displaced away from the margins of the optic disc.3 Glial cells only support the pre-laminar portion of the optic nerve, which accounts for the visible swelling during an ION attack.4 The disc swelling may be sectoral or diffuse and may have accompanying peripapillary nerve fiber layer hemorrhages.
A physiological small optic disc that has a small optic cup is at greater risk for developing NAION because there is less room for displacement of the swollen nerve fibers. As a result, the nerve is predisposed to going into a cycle of ischemia and edema. Such manifestations lead to non-reversible altitudinal visual field loss that is most often located inferiorly.5
NAION occurs most frequently in patients between the ages of 45 and 65 years.5 Some 12% to 19% of NAION patients develop it in the fellow eye within five years.6 Vision loss secondary to NAION is a serious complication of CABG surgery.
The etiology of NAION following CABG surgery is not clear. Several perioperative risk factors may act synergistically to initiate an ischemic attack of the retrolaminar region of the optic nerve. Suspected risk factors include hypotension, cardiac arrhythmias, thromboemboli released during cardiopulmonary bypass (CPB), hypocoagulability of blood, tissue edema secondary to CPB-induced inflammation, anemia and prolonged duration of CPB.7
| Signs of Clinically Severe Vascular Disease |
|
Our patient had severe vascular disease (carotid stenosis) and a reduced postoperative hemoglobin level that could have contributed to his postoperative NAION. Additionally, an uncontrolled study found that smoking is a significant risk factor for developing NAION and that quitting actually reduces that risk (although other studies have disputed this assertion).9,10,11 Our patients significant history of smoking may have acted synergistically with other risk factors (i.e., severe vascular disease, low postoperative hemoglobin, anemia, CPB) to cause postoperative NAION (see Risk Factors for PION in Our Patient, below).
|
Risk Factors for PION In Our Patient | ||
| (+)LOW HEMOGLOBIN LEVEL | ||
| Hemoglobin | Pre-Op: 14.6g/dl | Post-Op: 9.5g/dl |
| (13.6 to 17.4 reference range) |
||
| (+)ANEMIA | ||
| Red blood cell count | Pre-Op: 4.75m/cmm | Post-Op: 3.84m/cmm |
| (4.47 to 5.83 reference range) |
||
| (+)SEVERE VASCULAR DISEASE | ||
| Carotid stenosis | Carotid Artery Duplex Examination | |
| Hemodynamically significant stenosis - 50-79% of left and right carotid arteries | ||
| (+)PRE-OP ANGIOGRAM | ||
| Performed three days prior to CABG surgery. | ||
| (+)CARDIOPULMONARY BYPASS | ||
| Potential sequelae of CPB: Release of thromboemboli, greater blood loss, increased inflammation. | ||
| (+)TOBACCO | ||
| two packs per day for 35 years. | ||
The potential risk factors for developing PION following CPB appear multivariable. CPB is used for CABG surgery to sustain blood flow while the heart is stopped during the revascularization procedure. This is also referred to as on-pump CABG surgery. The procedure requires cannulation and cross clamping of the aorta; this can introduce thromboemboli into circulation.7 Other complications secondary to CPB: increased inflammation and greater blood loss.
A study that looked at PION in both on-pump and off-pump cardiac surgeries found no instances of PION in off-pump surgeries.7 With the advent of newer technologies that enable greater use of off-pump CABG surgery, the frequency of PION may be reduced.
Although there is currently no treatment for NAION, educate your patients about the possible in-creased risk of developing it in the fellow eye due to smoking. Also, take monocular precautions, and prescribe polycarbonate glasses for the protection of the fellow eye.
Coronary heart disease is a major public health issue. So, you must understand the complications associated with CABG surgery and educate patients undergoing CABG surgery about the risks of perioperative visual loss. Also, recognize and detect perioperative risk factors (i.e., severe vascular disease, postoperative hemoglobin concentration, preoperative angiogram) that may contribute to the onset of PION.
Dr. Anthony currently practices at the Louis-Stokes VAMC in Cleveland, OH. He lives in Shaker Heights, OH.
1. American Heart Association. Heart disease and stroke statistics 2005 Update. Chapter 3; 10-13
2. Arnold A. Pathogenesis of nonarteritic anterior ischemic optic neuropathy. J Neuro-Ophthalmol 2003, 23(2): 157-63
3. Foroozan R, Salvino P. How to uncover the cause of the swollen optic nerve. Rev Opth 2001 Jun;18(6): 93,94,99
4. Younge BR. Anterior ischemic optic neuropathy. www. emedicine.com/oph/topic161.htm (Accessed July 15, 2005)
5. Kanski J. Clinical ophthalmology: A systematic approach. 4th ed. Oxford: Butterworth-Heinemann, 1999:593-4.
6. Beck RW, Hayreh SS, Podhajsky PA, et al. Aspirin therapy in nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 1997;123:2127.
7. Kalyani S, Miller NR, Dong LM, et al. Incidence of and risk factors for perioperative optic neuropathy after cardiac surgery. Ann Thorac Surg 2004 Jul;78(1):34-7
8. Nuttall G, Garrity JA, Dearani JA, et al. Risk factors in ischemic optic neuropathy after cardiopulmonary bypass: A matched case/control study. Anesth Analg 2001 Dec;93(6): 1410-6
9. Chung SM, Gay CA, McCrary JA. Nonarteritic ischemic optic neuropathy. The impact of tobacco use. Ophthalmology 1994 Apr; 101(4): 779-82
10. Solberg Y, Rosner M, Belkin M. The association between cigarette smoking and ocular diseases. Surv Ophthalmol 1998 May-June:42(6): 535-47
11. Jacobson D, Vierkant R, Belongia E. Nonarteritic anterior ischemic optic neuropathy: A case-control study of potential risk factors. Arch Ophthalmol 1997 Nov; 115: 1403-07

