 A 62-year-old black male, who was receiving care for primary open-angle glaucoma, presented urgently with a new and unrelated problem. He reported that, approximately one week earlier, he suddenly experienced horizontal double vision, which was worse when looking to the right. When he looked straight ahead, the double vision was less evident. Also, his symptoms seemed to disappear when looking left. Further, he believed that his right eye was turning in toward his nose. Finally, the patient noted an ache behind his right eye when the trouble first started.
A 62-year-old black male, who was receiving care for primary open-angle glaucoma, presented urgently with a new and unrelated problem. He reported that, approximately one week earlier, he suddenly experienced horizontal double vision, which was worse when looking to the right. When he looked straight ahead, the double vision was less evident. Also, his symptoms seemed to disappear when looking left. Further, he believed that his right eye was turning in toward his nose. Finally, the patient noted an ache behind his right eye when the trouble first started.
Because he had hoped the problem was temporary, he delayed coming in for evaluation. He stated that the double vision worsened over several days, but had since stabilized and was not improving, so he decided to return for a check-up.
Upon examination, the patient had a right abduction deficit that worsened in right gaze. Forced duction testing showed that the right eye could be moved further out and was not physically restricted or tethered. His best-corrected visual acuity was unchanged at 20/25 O.D. and 20/30 O.S., and his pupils were reactive without afferent defect.
Upon questioning, he denied dysphagia, dysphasia, headache, paresis and paresthesia. Cranial nerve testing revealed no additional deficits beyond the abduction deficit. His optic nerves were
glaucomatous, but not edematous.
At this point, we diagnosed the patient with a neurologically isolated right cranial nerve (CN) VI palsy. His medical history was significant for medicated hypertension and hypercholesterolemia. At the time of examination, his blood pressure measured 155/95mm Hg. Based upon the diagnosis and associated medical history, we believed vascular ischemia was the basis of the CN VI palsy.
What is CN VI Palsy?
A patient with isolated, unilateral, acute-onset CN VI palsy will present with horizontal, uncrossed diplopia, which worsens at distance in either right or left gaze––depending upon the involved eye. The patient will have an abduction deficit in the involved eye and either a noncomitant esophoric or esotropic posture.1,2 If the palsy is isolated, the patient will not experience visual acuity/field loss or any other neurologic problems. However, there may be some degree of head or retro-orbital pain present, which is dependent upon the cause.
There are three distinct demographic groups that develop CN VI palsy:
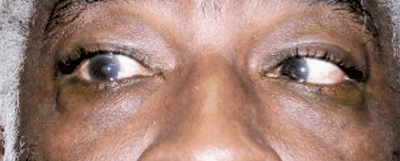
External view of the patient in right gaze. Note the abduction deficit in his right eye caused by CN VI palsy.
• Most patients who develop acute CN VI palsy are older than 50 years of age. This group often has a concurrent history of hypertension and/or diabetes (as did our patient).3-5 The peak incidence occurs in the seventh decade of life.6
• Children are also prone to develop CN VI palsy. The causes may range from benign (e.g., viral illness or trauma) to malignant etiologies.7-11
• The third group consists of young adults aged 20 to 50 years. These individuals are more likely to have neurologically complicated CN VI palsies involving additional cranial nerves (such as III and IV) or other neurological signs (such as ataxia or intention tremors).12,13 In contrast to older adults, vascular diseases such as diabetes and hypertension are uncommon in this group. Instead, more serious conditions such as central nervous system (CNS) mass lesions and multiple sclerosis typically are found.13-15
Additionally, because various cancers have been associated with CN VI palsy, patients may present with a pre-existing history of malignant disease. However, CN VI palsy may be the premonitory sign of cancer in some patients.
Managing CN VI Palsy
The most important consideration when managing acute onset CN VI palsy is identifying the causative factor in an efficient, cost-effective manner.
Doing so involves understanding common causes for each patient profile and palsy. In one large, population-based study, the four most common causes were idiopathic, hypertension alone, coexistent diabetes and hypertension, and trauma.6
A detailed medical history must be obtained as well as a neurologic examination upon initial presentation. Each case of CN VI palsy should be classified as traumatic or non-traumatic. Non-traumatic cases should be subdivided as neurologically isolated or non-neurologically isolated (e.g., either the patient only has a CN VI palsy or a CN VI palsy in addition to other neurological problems).6 Additionally, patients should be categorized into to one of three age-dependent management groups: children, young adults and older adults.6
A non-neurologically isolated CN VI palsy (involving other neurological structures concurrently) that shows any additional neurological signs (dysphasia, paresthesia, paresis, headache, disc edema or other cranial neuropathy) necessitates an MRI of the appropriate suspect area as well as cerebrospinal fluid analysis. Non-neurologically isolated CN VI palsies commonly are caused by cerebrovascular accidents that involve the pons, aneurysm (typically within the cavernous sinus) or neoplasm.6 While neurologically complicated CN VI palsy has a high likelihood of a serious cause (e.g., neoplasm), isolated adult CN VI palsy actually has a very low risk (2% in one series) of being caused by a neoplasm.6
• In children, sixth nerve palsy can be caused by a presumed viral etiology and has an excellent prognosis.9,10 However, nearly half of all CN VI palsies in children are due to neoplastic disease––notably pontine glioma.8,11 Thus, a pediatric neurologic evaluation and consultation is urgent in this age group, and the cause of the palsy should not be presumed benign.11
• In younger adults, CN VI palsy is likely to be caused by a serious underlying disease. In this group, CNS mass lesions account for 33% of CN VI palsies, and multiple sclerosis is responsible for another 24% of cases.13 Idiopathic CN VI palsies account for 13% of cases and vascular disease just 4%.13 It should be noted that CN VI palsy caused by CNS mass lesions in young adults often involve other cranial neuropathies and are not isolated. Thus, neuroimaging is highly recommended in this age group.
• In patients age 50 or older with an isolated sixth nerve palsy, a work-up for ischemic vascular diseases, such as diabetes and hypertension, should be performed.2-6 If the patient is over age 60, an erythrocyte sedimentation rate (ESR) and C-reactive protein should be ordered to rule out giant cell arteritis (if the clinical case dictates).
In cases of isolated CN VI palsy in older adults with a history of diabetes or hypertension, neuroimaging and other extensive evaluation can be deferred––unless the palsy progresses for more than one week, fails to improve over three months, or other neurologic complications develop. Ischemic vascular palsies typically progress over several days and may be no better in one week, but progression over two weeks warrants neurioimaging.6
Spontaneous recovery of CN VI palsy is common, especially if the etiology is idiopathic, traumatic or microvascular.3,4,6,13 Resolution of CN VI palsy typically is complete by three to six months––although some cases may take longer. CN VI palsies associated with CNS mass lesions tend to have a worse prognosis for spontaneous recovery.6,13 In cases where complete recovery does not occur, Fresnel prism correction may alleviate diplopia and visual discomfort. More aggressive therapy in non-remitting cases includes strabismus surgery or medial rectus injection with botulinum toxin (Botox, Allergan).14,15
In this case, we believed that the patient had an isolated CN VI palsy secondary to microvascular disease from hypertension. Because the patient believed that he had stabilized, neuroimaging was not ordered. However, we instructed the patient to call immediately if he noted any physical changes at all. The patient said that he was using an eye patch to ameliorate his symptoms, and we agreed that he should continue to do so.
At his most recent evaluation, 11 weeks after the onset of the double vision, he had completely resolved without complications and was happy. n
1. Goodwin D. Differential diagnosis and management of acquired sixth cranial nerve palsy. Optometry. 2006 Nov;77(11):534-9.
2. Staubach F, Lagrèze WA. Oculomotor, trochlear, and abducens nerve palsies. Ophthalmologe. 2007 Aug;104(8):733-46.
3. Richards BW, Jones FR Jr, Younge BR. Causes and prognosis in 4,278 cases of paralysis of the oculomotor, trochlear, and abducens cranial nerves. Am J Ophthalmol. 1992 May;113(5):489-96.
4. Rush JA. Paralysis of cranial nerves III, IV, and VI. Cause and prognosis in 1,000 cases. Arch Ophthalmol.1981 Jan;99(1):76-9.
5. Acaroglu G, Akinci A, Zilelioglu O. Retinopathy in patients with diabetic ophthalmoplegia. Ophthalmologica. 2008;222(4):225-8.
6. Patel SV, Mutyala S, Leske DA, et al. Incidence, associations, and evaluation of sixth nerve palsy using a population-based method. Ophthalmology. 2004 Feb;111(2):369-75.
7. Janssen K, Wojciechowski M, Poot S, et al. Isolated abducens nerve palsy after closed head trauma: a pediatric case report. Pediatr Emerg Care. 2008 Sep;24(9):621-3.
8. Broniscer A, Laningham FH, Sanders RP, et al. Young age may predict a better outcome for children with diffuse pontine glioma. Cancer. 2008 Aug 1;113(3):566-72.
9. Cohen HA, Nussinovitch M. Benign abducens nerve palsy of childhood. Pediatr Neurol. 1993 Oct;9(5):394-5.
10. Vallée L, Guilbert F, Lemaitre JF, et al. Benign paralysis of the 6th cranial nerve in children. Ann Pediatr (Paris). 1990 May;37(5):303-5.
11. Lee MS, Galetta SL, Volpe NJ, et al. Sixth nerve palsies in children. Pediatr Neurol. 1999 Jan;20(1):49-52.
12. Brinar VV, Habek M, Ozretić D, et al. Isolated nontraumatic abducens nerve palsy. Acta Neurol Belg. 2007 Dec;107(4):126-30.
13. Peters GB 3rd, Bakri SJ, Krohel GB. Cause and prognosis of nontraumatic sixth nerve palsies in young adults. Ophthalmology. 2002 Oct;109(10):1925-8.
14. Kao LY, Chao AN. Subtenon injection of botulinum toxin for treatment of traumatic sixth nerve palsy. J Pediatr Ophthalmol Strabismus. 2003 Jan-Feb;40(1):27-30.
15. Holmes JM, Leske DA, Christiansen SP. Initial treatment outcomes in chronic sixth nerve palsy. J AAPOS. 2001 Dec;5(6):370-6.

