Making the DiagnosisExplore these other articles featured in our 9th Annual Diagnostic Skills & Techniques Issue.
|
Have you ever had “analysis paralysis’’—that feeling of having too many choices and not knowing which to choose? I have that feeling every time I stop for ice cream at my local shop with over 75 flavors. I also feel that way about the number of diagnostic tests we have available for dry eye. Are there too many? Do I need to perform them all? Which results are going to give me the most salient information to manage each patient’s condition? Depending on your practice, you may have many or only a few diagnostic tests and tools available. Sometimes you’re not limited by availability but rather by chair time. You may limit yourself to just one or two dry eye tests per visit and think that’s all you need.
Here, we help you sort and tier your dry eye diagnostic tests in order to best position them for your specific practice and patient.
What are our “workhorse tests” that can and should be done on every potential dry eye patient to help you make a diagnosis? I label those Tier 1. What are additional procedures that can elevate your dry eye practice and increase the specificity of your diagnosis and management, but which may not be available to or convenient for everyone (Tier 2)? Finally, what are some high-tech tests being done in some of the country’s most high-touch dry eye practices today (Tier 3)?
The aim with Tier 1 tests in my mind is multifactorial; in theory, these are tests that are easy to perform, low-cost or free to use and available to most practitioners without upfront investment in much equipment.
Tier 2 tests may require more investment and skill to perform but they can help refine diagnosis and improve treatment.
Tier 3 tests are high-touch diagnostics that require an initial outlay of major capital or may only be used in the management of severe/advanced dry eye.
The current Tear Film and Ocular Surface (TFOS) Dry Eye Workshop (DEWS) II definition of dry eye, released in 2017, describes the presence of ocular surface symptoms and signs of dry eye. A dual strategy of quantifying symptoms paired with strategic use of diagnostic, point-of-care testing, can help better determine the etiology of your patient’s symptoms. This approach can shape your diagnostic testing selections or highlight additional treatment options at follow-up visits. For example, the TFOS DEWS II report distinguishes between aqueous-deficient and evaporative types of dry eye and each type may necessitate varying management strategies.1
It is also important to remember that these diagnostic tests can affect the results of one another if performed in sequence. Though many are noninvasive in nature, they still involve alterations in blinking patterns or bright illumination, which can affect results. As such, it is recommended that any dry eye diagnostic test be performed in a sequence from least to most invasive.1,2
 |
A 72-year-old female patient with severe conjunctivochalasis and recalcitrant ocular surface disease. She was eventually referred for conjunctival resection. Click image to enlarge. |
Tier 1: Workhorse Tests For Everyone
These are the first set of tests that should be available in every practice. They are easy to perform, relatively low-cost and should be conducted on all patients showing dry eye symptoms.
SYMPTOM SURVEYS
When considering any diagnosis of dry eye, start with symptoms. We generally think of case history as the best place to discuss symptoms; however, patient responses can be variable and inaccurate at times, especially if clinicians do not ask clear, pointed questions. Validated surveys are one key to clinching the initial diagnosis when patients present with one or more symptoms consistent with dry eye. The surveys can be repeated over time and there are several designs to fit your patient’s needs.
The DEWS II Diagnostic Methodology report recommends performing diagnostic testing if the screening Dry Eye Questionnaire (DEQ-5) or the Ocular Surface Disease Index (OSDI) confirms that a patient might have dry eye disease. OSDI is the most widely used survey in clinical trials and the standard by which other questionnaires are judged.3 OSDI measures frequency of symptoms, environmental triggers and vision-related quality of life. The screening cut-off is an OSDI score of ≥13; further, the scoring is categorized as mild (13 to 22 points), moderate (23 to 32 points) and severe (≥33 points). The average healthy population’s mean OSDI score is 9.6.3
If you’re looking for a streamlined survey, you may opt for the DEQ-5 (or for contact lens wearers, the CLDEQ-8).4,5 The DEQ-5 is short and easy to administer. It discriminates well between patients with and without dry eye, as well as between dry eye patients with and without Sjögren’s syndrome (SS).6 In general, DEQ-5 scores >6 suggest dry eye and scores >12 may indicate further testing is necessary to rule out SS dry eye.
One other popular survey option is the Standard Patient Evaluation of Eye Dryness (SPEED) test.7 The SPEED survey appears to be of superior value in evaporative dry eye.8 In general, patients with no symptoms score zero on SPEED. Those scoring 1 to 9 are categorized as mild to moderate and those scoring ≥ 10 are categorized as severe.9
Since symptom surveys can vary in design and target audience, you may wish to do more research before deciding which is the best fit for your patient base. Repeating the survey at each visit can assess improvement—or lack thereof—in symptoms over time as therapy is initiated, continued or discontinued.
While symptom surveys can assist practitioners in identifying patient symptoms, relying solely on this approach can exclude a large number of patients, as signs and symptoms are often uncorrelated.1 We must include other diagnostic tests in conjunction with symptom surveys to increase our ability to diagnose a condition as ubiquitous as dry eye.
According to the definition and classification subcommittee of TFOS DEWS II, “tear film instability” is part of the revised definition of dry eye.10 Many ways of assessing tear film stability have been described, including tear break-up time (TBUT) using fluorescein or noninvasive measures, thermography, variability in tear osmolarity and tear evaporation rate. It should be noted that thermography and tear evaporation rate are not well-established clinical techniques. Each test of tear film stability also requires meticulous performance and attention to exogenous factors, such as humidity or temperature, which may affect results. Overall, tear film stability testing is highly variable in outcomes.1 Any tear film stability test should be performed prior to other invasive tests, such as eyelid manipulation or staining of the ocular surface.1
TBUT is probably the most familiar and widely used test to evaluate tear film stability in clinical practice. Given the inherent variability of the measurement, three measurements are often averaged.
One common method for TBUT assessment involves the application of sodium fluorescein (NaFl) using a dye-impregnated strip. The clinician should wet the NaFl strip with a drop of saline, taking care not to touch the bottle tip to the strip. The excess saline is flicked off of the strip and the strip is laid flat on the temporal bulbar conjunctiva. It is recommended to wait between one and three minutes before evaluation to allow the NaFl to equilibrate with the tear film. Before assessment, the patient is instructed to blink naturally three times and then refrain from blinking. The clinician can begin timing until the first dark spots (or break-up) are detected in the tear film. A break-up time of ≤10 seconds indicates tear film instability and ≤5 seconds more definitively suggests a diagnosis of dry eye.1
It should be noted that within the DEWS II Diagnostic Methodology Report, a noninvasive measure of tear break-up time (NITBUT) is preferred over the standard fluorescein-tear breakup time described above.1 Interestingly, results for the two techniques are well correlated, but subjective aspects of FTBUT make it difficult to repeat among clinicians. Best practices for NITBUT include implementation of videokeratoscopy for automated measurement, along with repeating the measurement three times to determine an average result. The instrumentation needed to perform NITBUT is not available in every office and thus, automated NITBUT may be relegated to Tier 3 testing in such scenarios. See also the discussions of Schirmer and phenol red thread testing below.
 |
|
A cobalt blue filter can highlight more subtle areas of staining, as in this patient who presented with foreign body sensation. Click image to enlarge. |
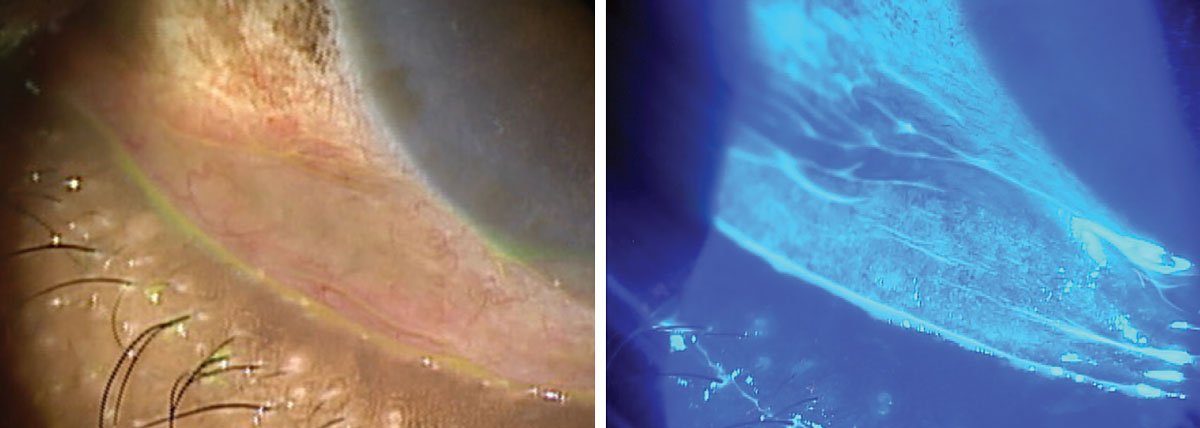
OCULAR SURFACE STAINING
The TFOS DEWS II definition of dry eye implies that dry eye can occur without ocular surface damage. However, ocular surface staining is still often a hallmark inclusion criterion for dry eye studies and instilled dyes are employed extensively in the diagnosis and management of dry eye. This testing can be repeated at each follow-up visit to gauge improvement across the cornea, conjunctiva and lid margin. The distribution of punctate staining across the cornea may also provide etiological clues to astute clinicians to help identify conditions such as lagophthalmos or meibomian gland dysfunction.
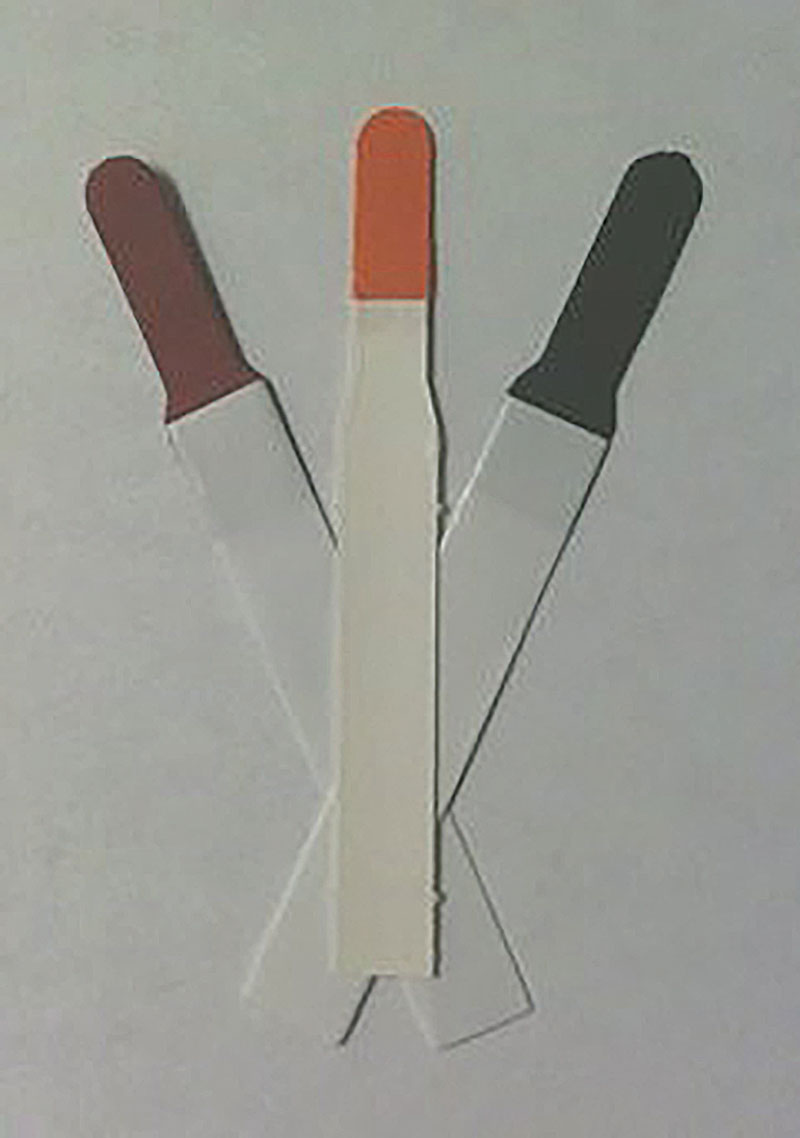 |
Three commonly used vital dyes are rose bengal, NaFI and LG. Click image to enlarge. |
Three frequently used ophthalmic dyes are NaFl, lissamine green (LG) and rose bengal. The latter has been shown to sting upon instillation, induce reflex tearing and be toxic to human corneal epithelial cells. Thus, it is recommended to use lissamine green, which is less toxic but just as well tolerated as NaFl.1
Best practices for use of these ophthalmic dyes include proper instillation (typically temporal to avoid confounding damage to the conjunctiva and lid margins), use of sequential staining with more than one paper strip to increase the likelihood of observing ocular surface damage and proper recording of results in order to monitor changes over time.1
When assessing corneal surface staining, many scales are available including the van Bijsterveld Score (whole corneal staining intensity graded on a scale of 0 to 3; added to conjunctival staining score), NEI Workshop Guidelines (dividing the cornea into five sectors, scoring each on a scale of 1 to 3), Oxford Staining Score (a 0 to V grade, depending on the intensity of staining displayed) and the Ocular Staining Score, which quantifies corneal staining “dots” graded on a scale of 0 to 3:1,11
grade 0: 0 dots
grade 1: 1 to 5 dots
grade 2: 6 to 30 dots
grade 3: >30 dots
Using clinical grading scales can improve the repeatability of corneal and bulbar conjunctival assessment.
BULBAR REDNESS ASSESSMENT
Conjunctival injection is the most common clinical sign suggesting ocular surface inflammation. Most likely, you are already completing this straightforward assessment during a routine slit lamp evaluation on each patient. Signs of ocular surface inflammation include a bulbar redness score of grade 2 or more (normal=grade 1 or less). Of course, other etiologies for bulbar redness should be ruled out, such as allergic conjunctivitis or infection, prior to determining the inflammation could be related to dry eye. Any degree of conjunctivochalasis should also be assessed at this time, as that can be a contributing factor to dry eye symptoms.
Similar to NITBUT, there are automated measures of bulbar redness which can be obtained using a videokeratoscope, which would land it in the Tier 3 category, as these are usually in the same unit as a meibographer. Such objective measurements are more repeatable and reliable than subjective grading, especially when performed by multiple observers.12 Bulbar redness assessment should be repeated at each visit and assessed prior to any ocular surface staining, drop instillation or other invasive tests.
BLINK LID AND CLOSURE ANALYSIS
To assess lid closure, have your patient semi-reclined and place a transilluminator against their relaxed, closed, outer upper eyelids. The amount of light seen in the lid area between the lashes in the nasal, central and temporal sections of eyelids can be quantified on a scale of 0 to 3:
0=no light
1=minimal
2=moderate
3=severe
Any score greater than zero suggests some level of insufficient lid seal, which correlates to dry eye symptoms upon waking.13
The insufficient seal identified by Korb-Blackie testing differs somewhat from the more obvious nocturnal lagophthalmos, where the patient has visible incomplete closure of the eyelids while asleep. It can be useful to ask patients if they have ever been told they sleep with their eyes open to determine whether nocturnal lagophthalmos is present. This feature, in addition to inferior interpalpebral conjunctival staining (exposure keratopathy) and lid laxity upon gentle closure, can help clinch the diagnosis.
Blink characteristics may also play a role in dry eye symptoms. With the increase in digital device use, blink rates are reduced, which can exacerbate symptoms. One simple test to determine a reduced blink rate is to watch the patient use a digital device for 15 seconds, counting the number of blinks. Once 15 seconds have passed, multiply your result by four to get the blink rate per minute. If it is less than 12 to 15 blinks per minute, your patient may benefit from blink training to increase their blink rate. This simple test can help bring attention to a patient’s lack of blinking, which, in turn, affects their tear film stability.
LID MARGIN/MEIBOMIAN GLAND EVALUATION
In conjunction with the discussion of ocular surface staining using vital dyes above, staining of the lid margin and lid wiper with NaFl and LG can also give information regarding dry eye and ocular surface damage during a slit lamp evaluation. The presence of lid wiper epitheliopathy (LWE) may be associated with dry eye symptoms and is measured by repeat instillation of LG. An LG strip is wet, with the drop retained for five seconds to elute the dye. A drop from the strip is instilled in the temporal conjunctiva. This is repeated after about five minutes with a second strip and the staining is evaluated with white light in the slit lamp three to six minutes later. LWE is considered present if there is more than 2mm (in length) of staining after instillation of LG or if the staining exceeds 25% of the sagittal width (excluding Marx’s line).1
Diagnostic meibomian gland evaluation and expression can be employed in the course of a slit lamp evaluation using either a thumb, cotton swab or a device that applies a standardized pressure to the lid margin, such as the Meibomian Gland Evaluator (Johnson & Johnson Vision). Using such a device, one can apply pressure—equal to that of a blink—to the outer skin of the eyelid while visualizing the secretions from the meibomian gland (MG) orifices. The device is applied to the nasal, central and temporal lid, applying pressure to approximately eight glands at a time.
The number of MGs out of eight yielding liquid secretion is the “meibomian gland yielding liquid secretion”score.14 The quality of the meibum expressed is assessed on a scale ranging from zero to three; zero is no expression, one is paste-like expression, two is turbid expression and three is normal expression of clear, thin, oil-like meibum. This diagnostic MG function evaluation can be repeated at each visit to gauge change over time from evaporative dry eye.
These diagnostic MG expression procedures contrast with the therapeutic expression procedures that are often used in treatment of MG dysfunction. The therapeutic procedures may involve eyelid warming followed by gland expression with the application of specialized forceps or paddles, or use of a dedicated therapeutic system that applies heat and pressure to the lids simultaneously. In these therapeutic expression procedures, the pressure applied to the lids exceeds that of the diagnostic expression.
Tier 2: Ancillary Testing For Refinement/Precision
Although these require an investment, they take your dry eye practice to the next level by refining diagnosis and improving treatment.
This has been in use to assess aqueous tear volume since the early 1900s.15 The most commonly used version for dry eye is Schirmer I, which measures both basal and reflex tear production. No anesthetic is used, and a Schirmer paper strip is placed on the lower eyelid margin for a duration of five minutes. Any value <10mm of wetting is considered abnormal. Some parties do not recommend Schirmer testing in the diagnosis of dry eye due to its variability and lack of accuracy; however, it is still recommended for the diagnosis of SS (where <5mm wetting is generally considered suspicious).15
An alternative to Schirmer testing is phenol red thread testing (PRTT), which is generally more comfortable for patients and faster to perform in only 15 seconds. The small yellow-colored thread changes to light-red in color once exposed to tears, and no topical anesthetic is necessary. The cut-off is also 10mm of wetting, with less wetting indicating likely dry eye. However, studies have suggested mixed results in terms of validity when comparing Schirmer testing with PRTT.16–18
 |
|
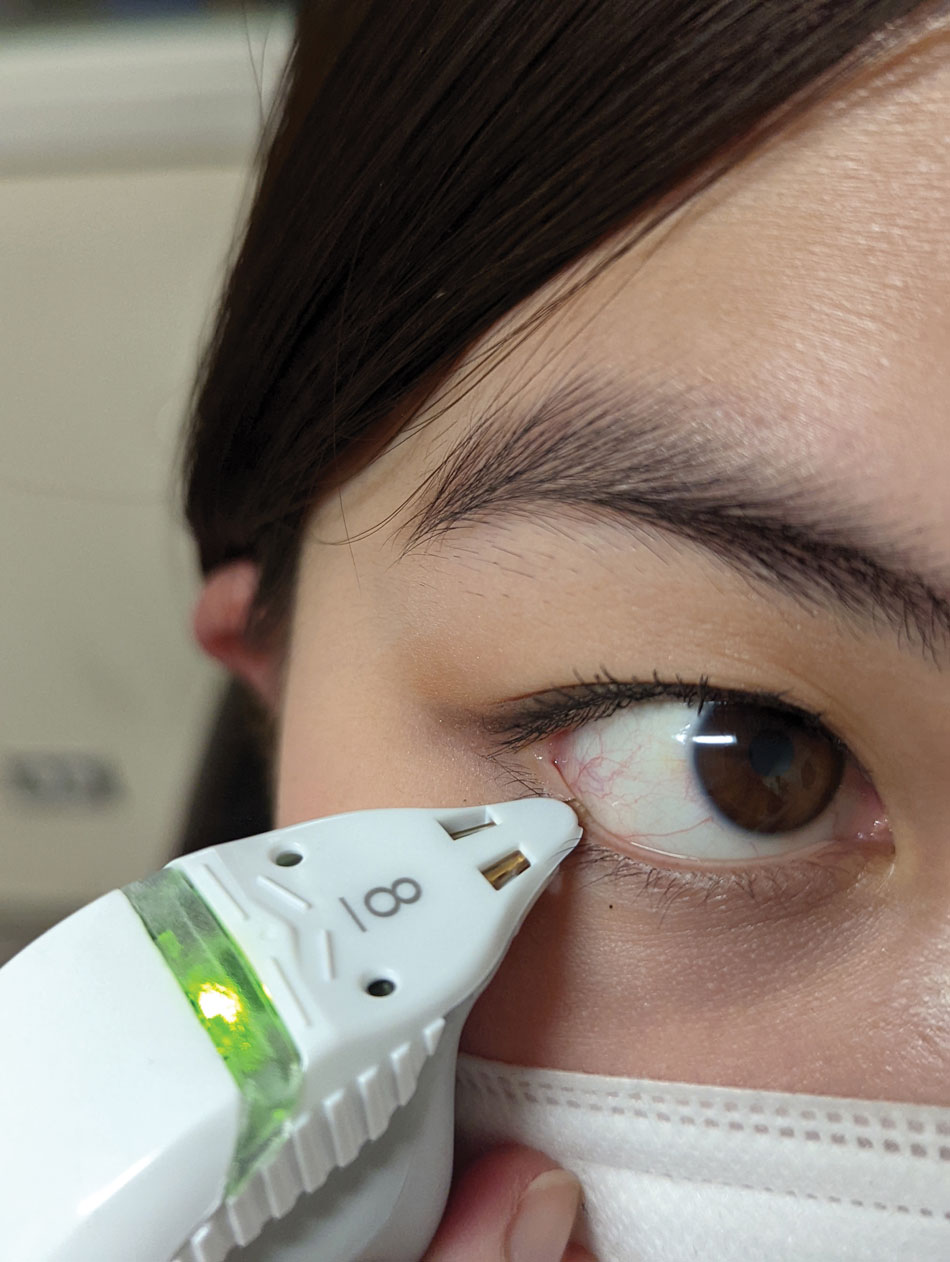
This patient was tested using a tear osmolarity system and the result showed elevated osmolarity level of 312 mOsm/L. It is important not to manipulate the lid while taking the sample. Click image to enlarge. |
TEAR OSMOLARITY
Though this test is found in Tier 2, tear osmolarity measurements should be performed after symptom survey and NITBUT testing but before ocular surface staining and other more invasive procedures. In a clinical setting, objective and quantitative measurement of tear osmolarity is easily obtained using a point-of-care test (TearLab). The measurements should always be taken with a calibrated device that has been at room temperature for at least 30 minutes prior to testing.
While gathering the sample, care should be taken not to pull the eyelid down or away from the globe. The test card tip should make contact with the lower tear meniscus at the lid margin. A positive result is considered to be >308 mOsm/L but can also occur with an interocular difference of >8 mOsm/L.19,20 Testing can be repeated as often as every dry eye-related visit, but may only need to be performed annually if the patient is stable.
MMP-9 TESTING
Matrix metalloproteinase-9 (MMP-9) testing is now easily available in a point-of-care device that can assay levels in just ten minutes (InflammaDry, Quidel) and thus joins tear osmolarity in Tier 2. The test is non-specific to the source of ocular inflammation and produces an easy-to-read positive (levels above 40ng/mL) or negative (levels below 40ng/mL) result. Similar to tear osmolarity, MMP-9 testing can be repeated as often as every dry eye-related visit but may only need to be performed annually if the patient is stable. It can also be used to help guide steroid therapy, which should reduce MMP-9 levels in tears.
TEAR MENISCUS HEIGHT (TMH) ASSESSMENT
This measurement is a generally accepted method to assess tear film volume. It can be performed using several methods, including with the aid of digital imaging or instrumentation. In the slit lamp, the TMH can be assessed by comparing its thickness to the lower lid margin or to a known size of slit beam. However, studies have shown greater repeatability using optical coherence technology (OCT) to image the TMH, which potentially places this automated method into Tier 3.21
Overall, it is suggested that TMH is a powerful predictor of tear film insufficiency.15,22 Average values for TMH vary somewhat in the literature, from 0.2 to 0.7mm, with a lower cut-off for normal considered to be ≤0.1mm.23
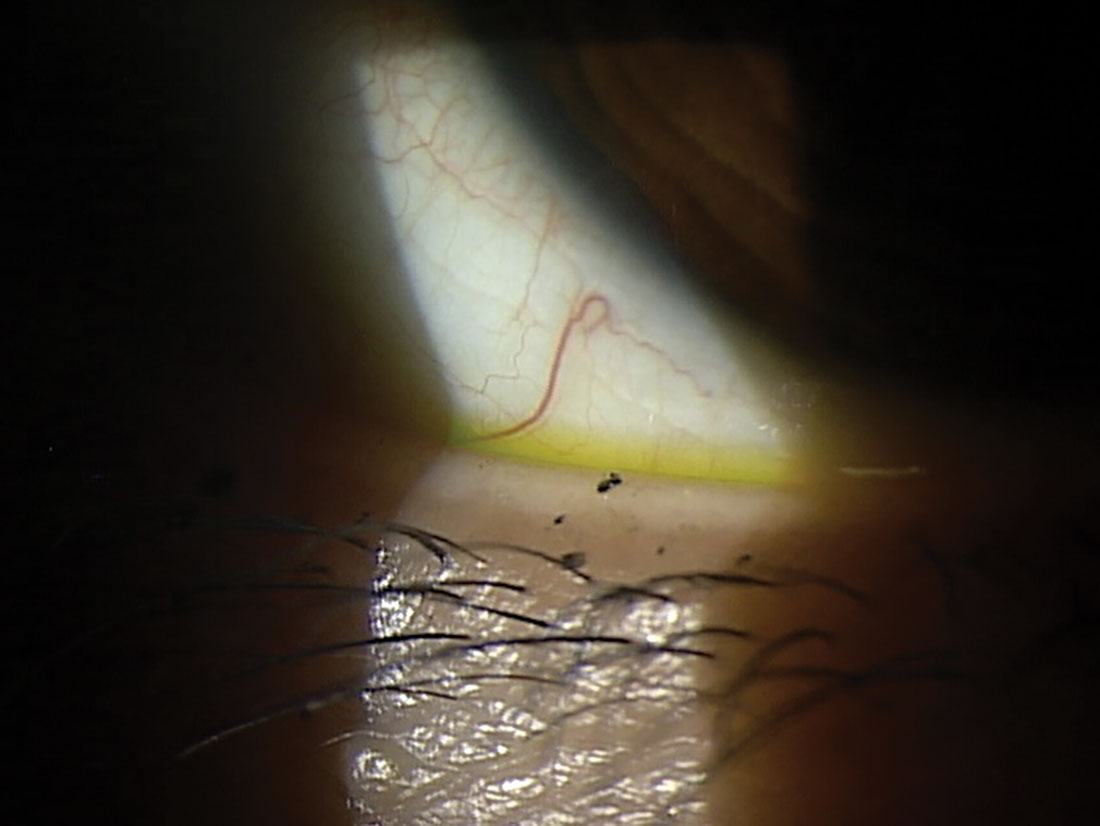 |
This patient’s tear meniscus is highlighted by the addition of NaFI. A cobalt blue filter can also assist with visualization. This patient has an adequate tear meniscus, but their ocular surface symptoms are exacerbated by makeup debris and systemic medications. Click image to enlarge. |
CORNEAL SENSTIVITY TESTING
With the commercial availability of cenegermin-bkbj ophthalmic solution (Oxervate), a recombinant human nerve growth factor for corneal healing in neurotrophic keratitis, corneal sensitivity testing has received more attention in recent years. Simple techniques for assessing corneal sensitivity have limited accuracy due to their subjective nature.
 |
|
The 0.2mm spot size on your slit lamp can be used to help estimate TMH. Click image to enlarge. |
The simplest technique involves touching the cornea gently with a wisp of cotton from a swab (alternatively, one could use a 3cm length of unscented, waxed dental floss or the corner of a tissue) to initiate a blink response.24,25 This low-cost approach is quick and easy to perform but lacks the quantitative results that could be gained through use of a formal measuring device such as the Cochet-Bonnet esthesiometer.
Such a device quantifies corneal sensitivity using a fine nylon filament which just touches the corneal surface. The longer the filament, the more flexible it is, indicating that more corneal sensation is present.24 Use of the Cochet-Bonnet may be more appropriately relegated to Tier 3 testing; however, the cotton swab approach is easily implemented by any clinician to rule out neurotrophic keratitis in patients with dry eye symptoms. It is recommended that corneal sensitivity be assessed in all four quadrants, along with the central cornea.
Tier 3: High-Touch Expert Level Testing
While costly, these cutting-edge tests are necessary for the most severe and advanced dry eye cases. Offering these elevates your dry eye practice to the highest level and can make your practice stand out in your area.
MEIBIOGRAPHY (MEIBOMIAN GLAND IMAGING)
While a basic form of meibography or meibomian gland imaging can be performed using a transilluminator on the everted lid, the technique has evolved to non-contact direct illumination of the glands using infrared illumination and computerized analysis of gland morphology.26,27 The MGD Workshop proposed that patients 20 years and younger should have no MG dropout and those 20 years and older may have less than or equal to 25% dropout.14
 |
|
Corneal sensitivity can be quantitatively measured using an esthesiometry instrument with a nylon filament. Click image to enlarge. |
BLOODWORK
Any eyecare practitioner can directly order or refer dry eye patients for bloodwork to rule out dry eye-related conditions such as rheumatoid arthritis, the seronegative spondylarthropathies and Sjögren’s syndrome (SS). Specific to dry eye, we commonly order testing for these patients using a laboratory order form indicating the proper diagnosis code (most often, keratoconjunctivitis sicca not specified as SS) and tests to order.
The specific testing may differ depending on the patient but often includes classic SS biomarkers: SS-A/Ro, SS-B/La, antinuclear antibody and rheumatoid factor. Other novel biomarkers can also be ordered specifically in cases where SS is suspected, including autoantibodies to salivary gland protein-1, parotid secretory protein, and carbonic anhydrase VI. These novel biomarkers may allow for earlier diagnosis of SS.28
Sjö (Immco Diagnostics) is a diagnostic test kit for these novel biomarkers that became commercially available starting in 2014 but may currently be challenging to source. Thus, we have taken to ordering blood work with a request that the novel biomarkers also be tested in whatever way is preferred by the patient’s receiving laboratory. Blood work can be performed initially and may not need repeating. However, if a patient is negative for the biomarkers of SS, their dry eye progresses and clinical suspicion of SS remains high at a later date, it may be worth repeating the bloodwork to see if the values change.
In vivo confocal microscopy (IVCM) can be used to assess a variety of anterior segment structures involved in dry eye including the cornea, conjunctiva and meibomian glands. This instrument lands in Tier 3, as it is not seen in most individual offices but rather at larger academic medical centers and tertiary care facilities.
To assist in diagnosing dry eye, IVCM can confirm an increase in mean corneal dendritic cell (DC) density above normal values—an indicator for active immune response or inflammation in the cornea that has been reported in dry eye patients.29 Aqueous-deficient dry eye patients have higher DC density when compared to evaporative dry eye patients, and aqueous deficient dry eye patients with related systemic immune disease showed higher DC density when compared with those dry eye patients with non-immune conditions.29,30 Alterations in corneal nerve length and density are additional biomarkers that are predictive for dry eye.29
INTERFEOMETRY
This sophisticated assessment of the tear film can be obtained via non-invasive imaging, the result of which assigns a predominant spectral color to the tear film lipid layer (TFLL) thickness. The reported thickness range of the normal TFLL is approximately 20µm to 160µm.14 Thinning of the TFLL has been reported in cases of both aqueous-deficient and evaporative dry eye, and has been shown to improve after punctal occlusion surgery.14,31 Varying tear interferometric patterns have been shown to identify differences in tear film kinetics among clinical subtypes of dry eye.32
EPITHELIAL THICKNESS MAPPING
This OCT-derived measurement is currently only commercially available on a few instruments from a single manufacturer in the United States (Optovue). The technology gives a non-contact, quantitative measurement of the corneal epithelium and stroma using ultra-high resolution anterior segment OCT. One study suggests superior thinning is characteristic of dry eye while another suggests central thickening. Overall, significant corneal epithelial irregularity is the hallmark finding.33–35
Other expert-level testing strategies for dry eye that are not currently in commercial use include impression cytology and tear ferning.
Impression cytology involves removing cells from the first to third most superficial layers of the conjunctival epithelium via application of cellulose acetate filters or biopore membranes. The cells are then analyzed by various methods, depending on the aim of the investigation, including microscopy, polymerase chain reaction and flow cytometry, among others.1
Tear ferning analysis involves drying tears on a glass plate to assess the crystallization (pattern of the tear fern) over a period of seven to ten minutes under normal room temperature and humidity.1 Healthy tear samples produce compact, dense ferning patterns, while dry eye samples show a fragmented or absent tear ferning pattern.36 It is possible that one day these technologies will make their way into our clinical practice.
 |
Tear osmolarity unit showing elevated osmolarity reading of 312 mOsm/L. Click image to enlarge. |
Takeaways
No matter which way you choose to stratify the diagnostic tests, the most important piece of dry eye management is to use some number of diagnostic tests in your workup. Pick and choose a few to embrace and implement in your practice. Repeat them and use those results to inform your management strategies and educate your patients.Over time, you may choose to implement more technologies that can improve your diagnostic capabilities and might even elevate you to “expert-level” territory. But even if you only have a slit lamp and your highly educated brain, you can still make a difference in the lives of patients with DED.
Dr. Sicks is an associate professor at ICO and a clinical attending in the Cornea Center for Clinical Excellence at the Illinois Eye Institute. She lectures and conducts research on specialty contact lenses.
| 1. Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15(3):539-74. 2. Foulks GN. Challenges and pitfalls in clinical trials of treatments for dry eye. Ocul Surf. 2003;1(1):20-30. 3. Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118(5):615-21. 4. Chalmers RL, Begley CG, Moody K, Hickson-Curran SB. Contact Lens Dry Eye Questionnaire-8 (CLDEQ-8) and opinion of contact lens performance. Optom Vis Sci. 2012;89(10):1435-42. 5. Chalmers RL, Keay L, Hickson-Curran SB, Gleason WJ. Cutoff score and responsiveness of the 8-item Contact Lens Dry Eye Questionnaire (CLDEQ-8) in a large daily disposable contact lens registry. Cont Lens Anterior Eye. 2016;39(5):342-52. 6. Chalmers RL, Begley CG, Caffery B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye. 2010;33(2):55-60. 7. Korb DR, Herman JP, Greiner JV, et al. Lid wiper epitheliopathy and dry eye symptoms. Eye Contact Lens. 2005;31(1):2-8. 8. Scaffidi RC, Korb DR, Greiner JV, Blackie CA. Lipid layer thickness and dry eye symptoms. Invest Ophthalmol Vis Sci. 2005;46(13):4444. 9. Blackie CA, Solomon JD, Scaffidi RC, et al. The relationship between dry eye symptoms and lipid layer thickness. Cornea. 2009;28(7):789-94. 10. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15(3):276-83. 11. Begley C, Caffery B, Chalmers R, et al. Review and analysis of grading scales for ocular surface staining. Ocul Surf. 2019;17(2):208-20. 12. Sorbara L, Simpson T, Duench S, et al. Comparison of an objective method of measuring bulbar redness to the use of traditional grading scales. Cont Lens Anterior Eye. 2007;30(1):53-9. 13. Blackie CA, Korb DR. A novel lid seal evaluation: the Korb–Blackie Light Test. Eye Contact Lens. 2015;41(2):98. 14. Tomlinson A, Bron AJ, Korb DR, et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):2006-49. 15. Holland EJ, Mannis MD FACS M, W. Barry Lee MDF. Ocular Surface Disease: cornea, conjunctiva and tear film. Elsevier Health Sciences. 2013. 16. Saleh TA, McDermott B, Bates AK, Ewings P. Phenol red thread test vs Schirmer’s test: a comparative study. Eye (Lond). 2006;20(8):913-5. 17. Vashisht S, Singh S. Evaluation of Phenol Red Thread test versus Schirmer test in dry eyes: a comparative study. Int J Appl Basic Med Res. 2011;1(1):40-2. 18. Masmali A, Alqahtani TA, Alharbi A, El-Hiti GA. Comparative study of repeatability of phenol red thread test versus Schirmer test in normal adults in Saudi Arabia. Eye Contact Lens. 2014;40(3):127-31. 19. Lemp MA, Bron AJ, Baudouin C, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011;151(5):792-8.e1. 20. Jacobi C, Jacobi A, Kruse FE, Cursiefen C. Tear film osmolarity measurements in dry eye disease using electrical impedance technology. Cornea. 2011;30(12):1289-92. 21. Niedernolte B, Trunk L, Wolffsohn JS, et al. Evaluation of tear meniscus height using different clinical methods. Clin Exp Optom. 2021;104(5):583-8. 22. Mainstone JC, Bruce AS, Golding TR. Tear meniscus measurement in the diagnosis of dry eye. Curr Eye Res. 1996;15(6):653-61. 23. Doughty MJ, Laiquzzaman M, Oblak E, Button N. The tear (lacrimal) meniscus height in human eyes: a useful clinical measure or an unusable variable sign? Cont Lens Anterior Eye. 2002;25(2):57-65. 24. Remington LA, Goodwin D. Clinical Anatomyand Physiology of the Visual System. Elsevier Health Sciences. 2011. 25. Dua HS, Said DG, Messmer EM, et al. Neurotrophic keratopathy. Prog Retin Eye Res. 2018;66:107-31. 26. Nichols JJ, Berntsen DA, Mitchell GL, Nichols KK. An assessment of grading scales for meibography images. Cornea. 2005;24(4):382-8. 27. Johnson LN, Diehl ML, Hamm CW, et al. Differentiating optic disc edema from optic nerve head drusen on optical coherence tomography. Arch Ophthalmol. 2009;127(1):45-9. 28. Belovay GW, Goldberg I. The thick and thin of the central corneal thickness in glaucoma. Eye (Lond). 2018;32(5):915-23. 27. Pult H, Nichols JJ. A review of meibography. Optom Vis Sci. 2012;89(5):E760-9. 28. Beckman KA, Luchs J, Milner MS. Making the diagnosis of Sjögren’s syndrome in patients with dry eye. Clin Ophthalmol. 2016;10:43-53. 29. Binotti WW, Bayraktutar B, Ozmen MC, et al. A review of imaging biomarkers of the ocular surface. Eye Contact Lens. 2020;46 Suppl 2(2):S84-105. 30. Kheirkhah A, Rahimi Darabad R, Cruzat A, et al. Corneal epithelial immune dendritic cell alterations in subtypes of dry eye disease: a pilot in vivo confocal microscopic study. Invest Ophthalmol Vis Sci. 2015;56(12):7179-85. 31. Hosaka E, Kawamorita T, Ogasawara Y, et al. Interferometry in the evaluation of precorneal tear film thickness in dry eye. Am J Ophthalmol. 2011;151(1):18-23.e1. 32. Arita R, Morishige N, Fujii T, et al. Tear interferometric patterns reflect clinical tear dynamics in dry eye patients. Invest Ophthalmol Vis Sci. 2016;57(8):3928-34. 33. Cui X, Hong J, Wang F, et al. Assessment of corneal epithelial thickness in dry eye patients. Optom Vis Sci. 2014;91(12):1446-54. 34. Kanellopoulos AJ, Asimellis G. In pursuit of objective dry eye screening clinical techniques. Eye Vis (Lond). 2016;3:1. 35. Abou Shousha M, Wang J, Kontadakis G, et al. Corneal epithelial thickness profile in dry-eye disease. Eye . 2020;34(5):915-22. 36. Masmali AM, Al-Qhtani S, Al-Gasham TM, El-Hiti GA, Purslow C, Murphy PJ. Application of a new grading scale for tear ferning in non-dry eye and dry eye subjects. Cont Lens Anterior Eye. 2015;38(1):39-43. |