Making the DiagnosisExplore these other articles featured in our 9th Annual Diagnostic Skills & Techniques Issue.
|
We all have our favorite tests, tools and practices, but just because things have always been done a certain way doesn’t mean they can’t be modified for the better. Some staples of the ophthalmic work-up may be considered outdated and need to be swapped for new, more up-to-date techniques.
In what perhaps may be more polarizing than the state of politics in America, we’re going to discuss many diagnostic tests and old-school ways of doing things that we think can be ditched or modified in optometry, and what is here to stay. Some of these may be controversial—opinions differ even among the four of us, as you’ll see in our roundtable below. Keep in mind that our comments shouldn’t be construed as the final word on these debates; our goal here is to start conversations, not end them. We invite you to follow along as we talk shop for a while!
Editor’s note: Readers are encouraged to share their own thoughts on specific topics, pro or con, in a letter to the editor. The panelists will consider any feedback they receive and weigh in with their takes in a future issue. Send your feedback to editor@reviewofoptometry.com.
Tools and Tests
Optometry is fortunate to have so many ways of assessing and documenting the status of the eye from both a structural and functional perspective. But when a more sophisticated test emerges, what should happen to the older protocols? Let’s examine several in a 2022 context.
Amsler Grid
Dr. Seng: In use since 1945, Amsler grid testing can detect functional disturbances in the central and paracentral region. The grid can be used to detect scotomas and metamorphopsia in the central 10° on all sides of fixation. Like the “facial Amsler grid” that is done during confrontation field testing, it has long been used as a screening tool, often completed by technicians, in patients at risk of conditions such as macular degeneration, toxic maculopathy, macular hole, retinal artery occlusion, central serous chorioretinpathy or homonymous loss from stroke. It is also used as a home screening tool so that patients can monitor their condition outside of the office, and while some might argue compliance is low and the test lacks sensitivity, it is certainly better than nothing.
However, with improved visual field protocols allowing for shorter and more reliable testing and most importantly, with SD-OCT testing now standard-of-care and available at the vast majority of eyecare offices, in-office Amsler testing can likely be retired when better tools are available. The Amsler grid has low sensitivity and is often somewhat misunderstood by patients, giving it low overall value as a clinical test.1 It is also likely that the results of testing, whether negative or positive, will not change the decision to move forward with additional and much more sensitive testing such as macular OCT scans. For this reason, I think Amsler grid testing can likely be removed from the routine preliminary testing protocol in most clinical settings.
Home Amsler grid testing’s role is a little less clear. Paper copies of grids are still often given out to patients for at-home monitoring, especially in conditions with high-risk of disease progression and where early detection can impact clinical outcomes. Studies have shown that home Amsler testing not only has low sensitivity, but also low compliance of patient use.1
Many studies are looking at alternative methods for home monitoring, including threshold Amsler grid, entoptic perimetry and preferential hyperacuity perimetry. These have been found to be more sensitive than Amsler grid testing and could conceivably be used by patients for self-monitoring, but require financial resources as well as technology that has not yet been developed and deployed on a widespread basis.1,2 They also do not necessarily solve the issue of poor patient sensitivity. So, while routine Amsler testing in-office has likely outlived its usefulness, home testing—while far from perfect—may still be the most practical method for improving early detection of vision changes indicative of disease progression in patients with high-risk macular disease.
Dr. Hicks-Hubbard: I’m anxiously waiting for a newer way to help patients monitor at home, but until then, the at-home Amsler grid is our best option. While it does have low sensitivity, we can still try our best with good patient education to help improve adherence to daily monitoring.
Dr. Weidmayer: Routine Amsler grid evaluation in-clinic is not a good use of time; however, it is occasionally useful for a patient to map out their area of distortion or scotoma to correlate functional abnormalities with structural findings. Don’t totally throw away your grid.
Dr. Taylor: It is a quick test to perform and can be helpful in some instances; so, maybe it’s not needed on every patient every time, but it has its place in the clinic. As far as the home Amsler grid goes, even the AOA Clinical Practice Guidelines for AMD acknowledge that Amsler often fails to detect abnormalities when used at home and emphasize carefully explaining how to monitor using Amsler to detect subtle changes. However, the guidelines still advise using it.
B-Scan
Dr. Taylor: Optical coherence tomography (OCT) gives providers extensive information on the posterior segment, and has been widely adopted as the go-to method of imaging this region. But what do you do when it is not visible? How can you quickly analyze a patient’s posterior segment in the presence of a tarsorrhaphy, opaque cornea, dense cataract, vitreous hemorrhage, hyphema or contraindication to dilation? B-scan ultrasonography permits two-dimensional visualization of the anterior and posterior segment when a direct view with the biomicroscope, indirect ophthalmoscopy or your OCT is not possible.
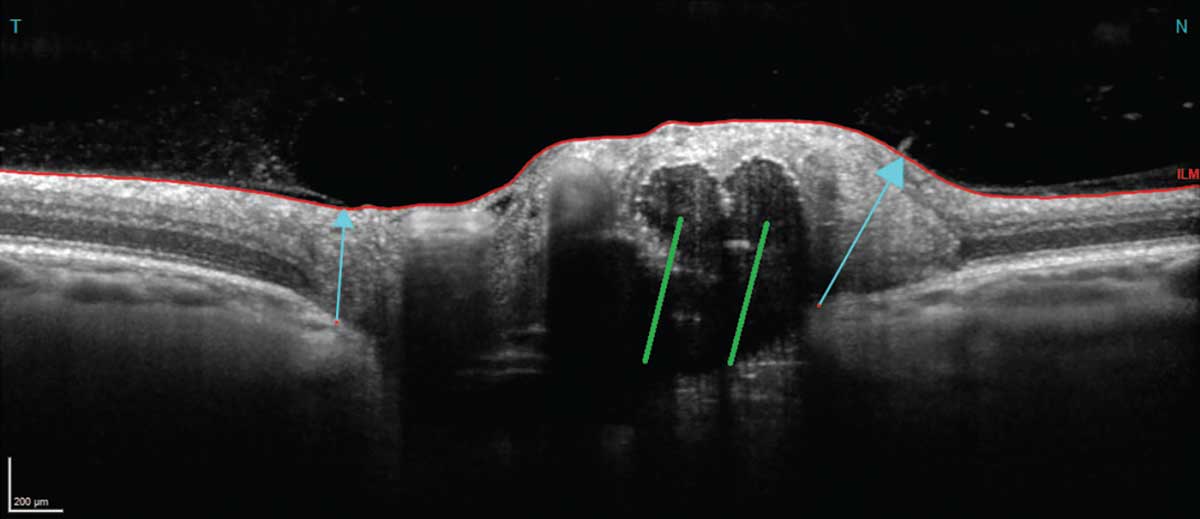 |
| An OCT line scan with the green lines annotating some visible optic disc drusen. Click image to enlarge. |
B-scan ultrasound imaging is accomplished by the transmission of high-frequency soundwaves. When the soundwaves hit intraocular structures, they may be absorbed, transmitted or reflected. Echo signals are detected by the probe, and a two-dimensional image is then reconstructed for viewing.
Many conditions can be easily diagnosed by performing a B-scan. These include optic nerve head drusen, papilledema, choroidal masses, retinal detachment, choroidal detachment and vitreous opacities, to name just a few. It is easy to perform, noninvasive to the patient, gives a quick and repeatable result, takes up minimal space and can be easily moved around the office. B-scan instrumentation is relatively inexpensive, with units beginning at around $7,500. In short: to conduct medical optometry, you need one!
Dr. Weidmayer: I mostly agree. You don’t need one…until you do. I don’t use mine often, but when you need it, you need it.
Dr. Hicks-Hubbard: The times I’ve needed the B-scan, I’ve been thankful for it! Like Dr. Taylor said, as a quick test that doesn’t require much space, the B-scan is handy to have in-office.
Dr. Taylor: I have at least one patient a month where I say, “I wish we had a B-scan.” I am old enough to remember when every cataract surgical patient had a B-scan as part of their pre-op testing.
Color Vision Testing
Dr. Taylor: This is an important baseline clinical test that should not be abandoned, as it can be beneficial in making occupational choices that require color differentiation. Also, congenital and acquired disorders that involve color vision can be identified and discerned by color vision screening techniques. The techniques used in color vision testing are fairly standardized and many different tests are commercially available. Which one is best for your office?
There are three broad categories of color vision tests: the Nagel anomaloscope, plate tests and arrangement tests. The anomaloscope is the definitive test for color vision deficiencies, but its expense, complexity of use and need for a skilled examiner makes it impractical for office use in most settings.
Plate tests have several advantages; mainly, they can be easily and quickly administered by minimally trained technicians. Color vision plates are inexpensive (under $200) and can be used on children and nonverbal individuals. Improper lighting can be a difficulty with administering the test (the plates were designed for use under a specific viewing conditions). Most importantly, classifying defects based on test performance is not based on an exact scoring criterion. The type or extent of a color vision deficit does not easily correlate to the number of mistakes made on the test. Color vision plates are a screening test used to expose red/green color vision deficits; they often do not adequately screen for blue/yellow acquired deficiencies.3
The (Farnsworth) D-15, the most common arrangement test, is inexpensive, easy to score and is accurate in determining color confusion. This test will take longer to administer than color plate testing. Manual dexterity is required as is some degree of patience and concentration, so it is probably not your first color vision test choice. However, it does have value in patients with especially high needs for color vision differentiation for professional or personal reasons.
Dr. Weidmayer: In my practice, color plates are useful when assessing optic nerve function (vs. dysfunction). One should always stay in the office. The D-15? Maybe keep that as some sort of abstract artwork; otherwise, I can live my life without it!
Dr. Taylor: D-15 discrimination is real, Sara!
Confrontation Visual Fields
Dr. Hicks-Hubbard: With automated perimetry and other forms of more thorough visual field testing becoming nearly ubiquitous, it would seem that confrontation visual fields (CVFs) would be of little use. Decades of research show us that confrontations aren’t sensitive and can fail to detect certain defects.4 So, why are we still performing them?
CVFs, while not perfect, are still of significant clinical value when performed properly. They are quick, simple to do and can provide immediate information regarding the visual pathway. For deep defects, such as a homonymous hemianopsia, confrontation CVFs are actually fairly reliable. Moreover, a provider can be confident that if they detect a defect on CVFs that it is real and truly present.4 By knowing the limitations of CVFs, a provider can continue to use them confidently among the other examination tools to assess a patient’s field of vision.
Dr. Taylor: Done correctly, confrontation fields give a wealth of information!
Dr. Seng: We tried for a short time to make frequency doubling technology take the place of confrontation fields; it didn’t work. We found too many false positives—defects on frequency doubling technology screening that then required Humphrey Visual Field testing, only to not be repeatable on that platform. CVFs are quick, simple and rarely result in false positive defects, thus they are still necessary (and much quicker) for screening purposes.
Fluorescein Angiography
Dr. Weidmayer: We have OCT-A! Who needs FA? The truth is: many patients do. OCT-A is amazing and has really broadened our ability to understand and manage disease. We are all enamored with it, and rightly so, in many ways. However, OCT-A does not show leakage or well-characterized vasculature with low flow like FA does.5
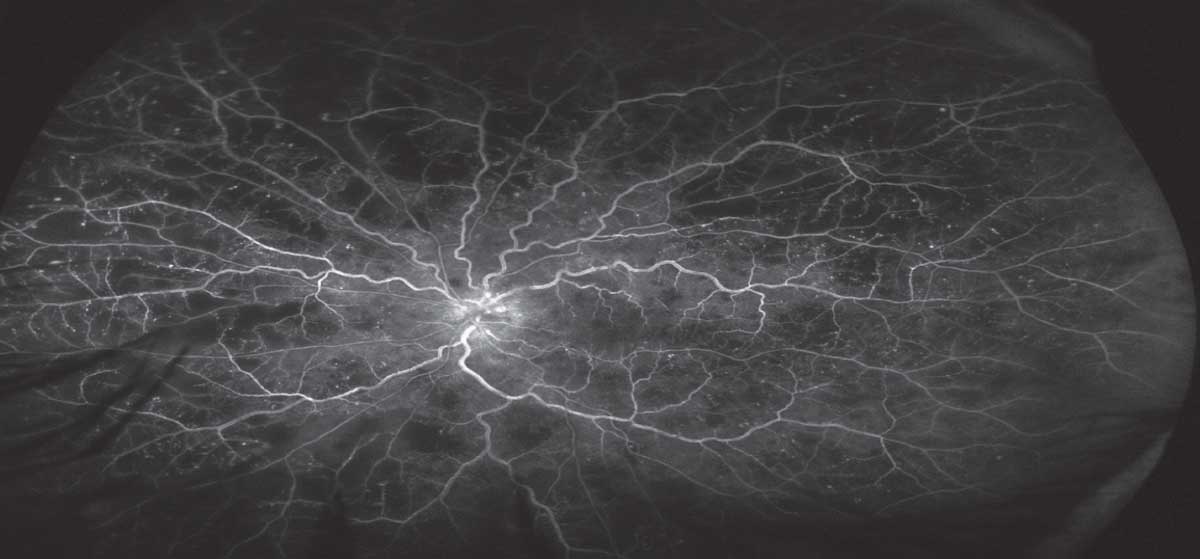 |
| This later-stage widefield fluorescein angiography (FA) in a patient with an ischemic central retinal vein occlusion shows several areas of capillary dropout, which were targeted with panretinal photocoagulation (PRP). Click image to enlarge. |
FA also gives us information about nearly the entire retina (depending on the field width of the camera), whereas OCT-A is limited typically to the macula or a section of the posterior pole at best. FA outperforms OCT-A for evaluating things like peripheral ischemia, which drives treatment decisions, such as PRP planning. So, while OCT-A is storming into clinical practice right now, it does not replace FA for many conditions.
Dr. Hicks-Hubbard: I agree! Being able to view in real time the perfusion of the fundus as well as neovascularization that becomes much easier to spot—FA is irreplaceable.
Dr. Seng: OCT-A may help decrease the frequency that FA is needed, just like OCT itself did; however, not yet to zero. Keep it!
Gonioscopy
Dr. Hicks-Hubbard: With anterior segment OCT (AS-OCT) being readily available, many practitioners may wonder why they should bother with proparacaine, a contact lens and a contact gel. AS-OCT can be performed by a trained technician, provides an objective finding and can be more comfortable for a patient sensitive to ocular touch.
However, multiple references support gonioscopy as the gold standard for assessment of the anterior chamber angle.6-8 While AS-OCT provides us with a trove of information, our gonioscopy lenses should not be replaced yet.9 Assessing any case of newly diagnosed glaucoma for angle recession, neovascularization of the angle (NVA), pigment dispersion syndrome, pseudoexfoliation or a small area of peripheral anterior synechiae all require a view of the iridocorneal angle that can only be achieved with gonioscopy. Additionally, compression of the angle can only be achieved with a contact lens, which is something that cannot be completed using AS-OCT.
It’s important to bear in mind that gonioscopy may be irreplaceable but AS-OCT can still provide unique clinical information. For instance, the patency of a laser peripheral iridotomy, as well as changes in iris configuration, can be imaged to provide a clinician further information regarding the angle.6 As with many ophthalmic advances, a combination approach may provide the greatest clinical benefit.
Dr. Weidmayer: I use gonio lenses all the time. We get a direct, 360-degree view of the angle; it’s invaluable for detection of NVA and I fully support Dr. Hicks-Hubbard’s comment about compression/dynamic gonioscopy, especially to differentiate whether an angle is phacomorphic or to determine if a laser peripheral iridotomy would even help widen an angle.
Dr. Taylor: Dr. Weidmayer, I agree with you 100%.
Pupil Testing
Dr. Taylor: The “swinging flashlight test” is one of the first diagnostic tests optometry students are taught. Pupillary abnormalities such as anisocoria, relative afferent pupillary defect (APD), findings suggestive of Horner’s syndrome, third nerve palsy or Adie’s tonic pupil need to be detected, documented and thoroughly investigated at the earliest time possible.
Rather than ditching it, delegate it. Do you have someone in your office besides yourself that can efficiently and accurately examine pupils? Having a well-trained staff will allow providers to see more patients and make office operations run more efficiently. Employees usually want to learn and develop their skills. Take the time to educate your technicians not only on how to check pupils, but also on the reasoning behind checking pupils. If you have trained your technicians to your satisfaction on pupillary testing, you then have to do the hard part: trust their results. Empower your technicians to practice, gain experience and learn from their mistakes.9
Dr. Weidmayer: I am very hesitant to agree. Missing a Horner’s syndrome or an APD, for example, could literally have life-or-death consequences.
Dr. Taylor: For sure, but a majority of our patients are dilated before I see them, which is the same for every optometrist I have worked with. Our technicians are trained that if there is any question, they should let the doctor know; they know not to guess.
Dr. Weidmayer: I suppose we all have to calculate our own risk vs. benefit and consider carefully who we are trusting to do this important evaluation.
Schirmer Testing
Dr. Hicks-Hubbard: Dry eye disease is a complex, multifactorial condition that is encountered frequently in primary eyecare examinations.10 Dry eye is complicated by the lack of a gold standard test for appropriate diagnosis. Optometrists have many tools at their disposal—osmolarity testing, phenol red test, Schirmer testing, tear break-up-time—as well as numerous patient questionnaires.11 These tests, among others, can tell optometrists whether to start treatment as well as what type of treatment will best aid the patient. But as technology and testing become more accessible, is it time to eliminate some of the originals, such as Schirmer testing?
A main fault with Schirmer testing is the lack of sensitivity and specificity in dry eye.11,12 Knowing when to use this test increases its clinical value. This testing is most useful when examining a patient with suspected Sjögren’s disease, but may not be as useful in the diagnosis of other forms of dry eye, such as evaporative.11 While Schirmer testing is not necessary on every patient with suspected dry eye, it still holds clinical value for a small subset with suspected severe aqueous-deficient dry eye disease.
Dr. Weidmayer: Schirmer’s is a fairly objective measure, so it may be more useful for quantifying improvement with treatment more than diagnosing dry eye in the first place. Overall, I say ditch it.
Dr. Taylor: I used to do it all the time until I figured out I never used the result in my clinical decision-making.
Nomenclature
As new research changes our conceptions and challenges our habits, it should do the same to the terminology we use. Are we keeping up with the literature?
Clinically Significant Diabetic Macular Edema (CSDME) Criteria
Dr. Weidmayer: This condition has long been defined by the Early Treatment Diabetic Retinopathy Study (ETDRS).13 We should all be able to rattle off the criteria, but is it clinically relevant anymore? The ETDRS evaluated the efficacy of lasers for macular edema (vs. monitoring), a treatment modality that is, in most cases, now far secondary to anti-VEGF injections. Our ability to image the macula with OCT has also changed our practice patterns and challenged the relevance of ETDRS’s CSDME criteria.14
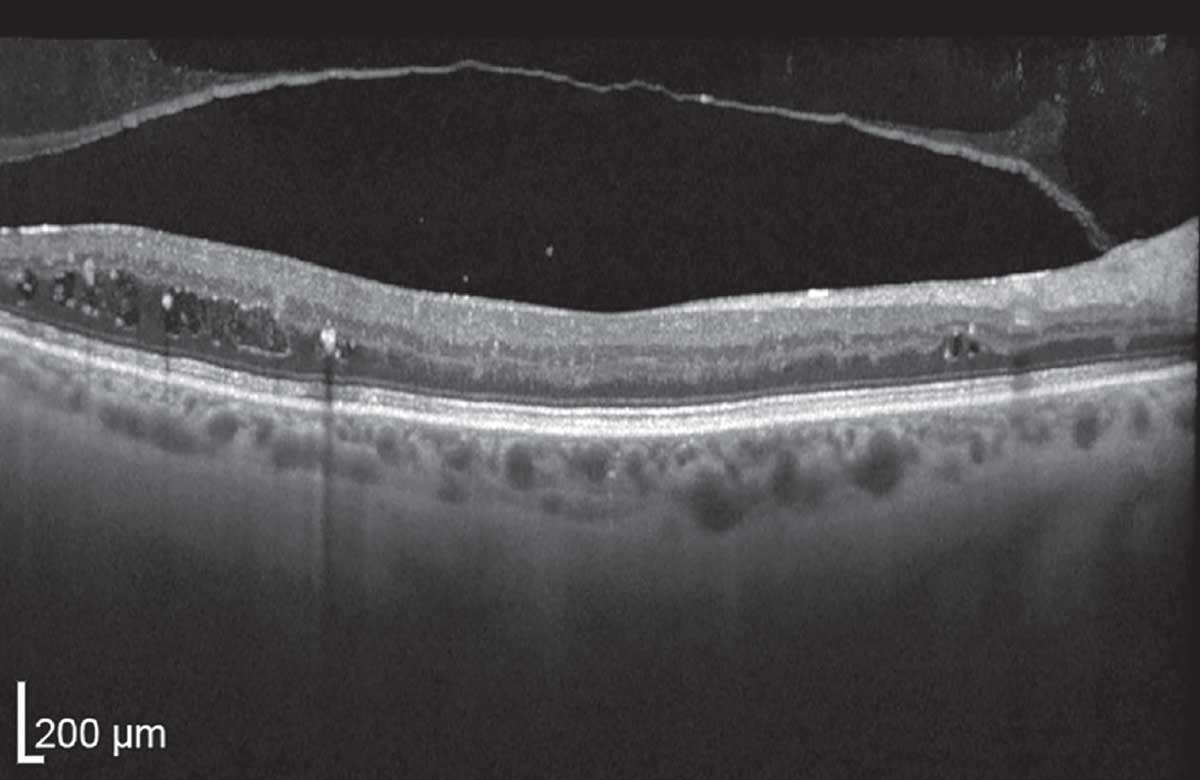 |
| This OCT shows non-center-involving but not visually significant diabetic macular edema (non-CI, NVS DME) with intraretinal fluid and exudates. This would qualify as CSDME per the ETDRS. Click image to enlarge. |
There has been a paradigm shift from this CSDME model to that of a center-involved (CIDME or CI-DME) vs. non–center-involved DME, and visually significant vs. not visually significant DME. When to inject anti-VEGF for DME has largely become a game of monitoring unless there is central-involving and visually significant DME, as visual and anatomic outcomes have not been shown to differ with or without treatment in those with CI-DME but good acuity (typically defined as at least 20/30).15,16 Certainly, this protocol varies per patient situation, but is broadly accepted.
It is still important to have universal criteria for the purpose of clinical grading of diabetic retinopathy, research protocols/outcomes, coding and so on, but from a clinical standpoint, the classic “CSDME” has largely gone by the wayside.
Dr. Hicks-Hubbard: It is hard to lose the definition known so readily by all eyecare providers, but as the primary treatment modality has changed, so has the treatment criteria. As retina specialists shift away from CSDME, so should optometrists.
Diabetic Retinopathy Staging
Dr. Hicks-Hubbard: The classification of diabetic retinopathy has been based upon seven standardized photographs since the late ’60s. While this classification and staging has evolved over time, a significant change hasn’t been made since the ETDRS.16 With so much time between then and now, are we overdue to adjust our staging?
It is essential to have universal criteria for diabetic retinopathy staging, but with recent technological advances, particularly the advent of ultra-widefield photography (UWF), it may be time to update our classification. With UWF photography, a larger portion of the retina can be photographed, reviewed, assessed and staged. When comparing UFW and the standard seven-field photograph series from the ETDRS, skilled readers demonstrate agreement in the grading of the severity of diabetic retinopathy. Furthermore, UWF has been shown to have the ability to image peripheral lesions previously not seen by the standardized seven-field photograph series.17 However, it is still unknown what this peripheral pathology can tell us about the progression of the disease.17
Despite the clear advances in imaging technology, the clear-cut definitions and photographs from the ETDRS are not obsolete; rather, we may be adding to them. As we can better image the retina, we may be able to adjust or augment our staging based on peripheral retinal pathology, which will allow us to better assess a patient’s risk of progression to more severe forms of diabetic retinopathy.
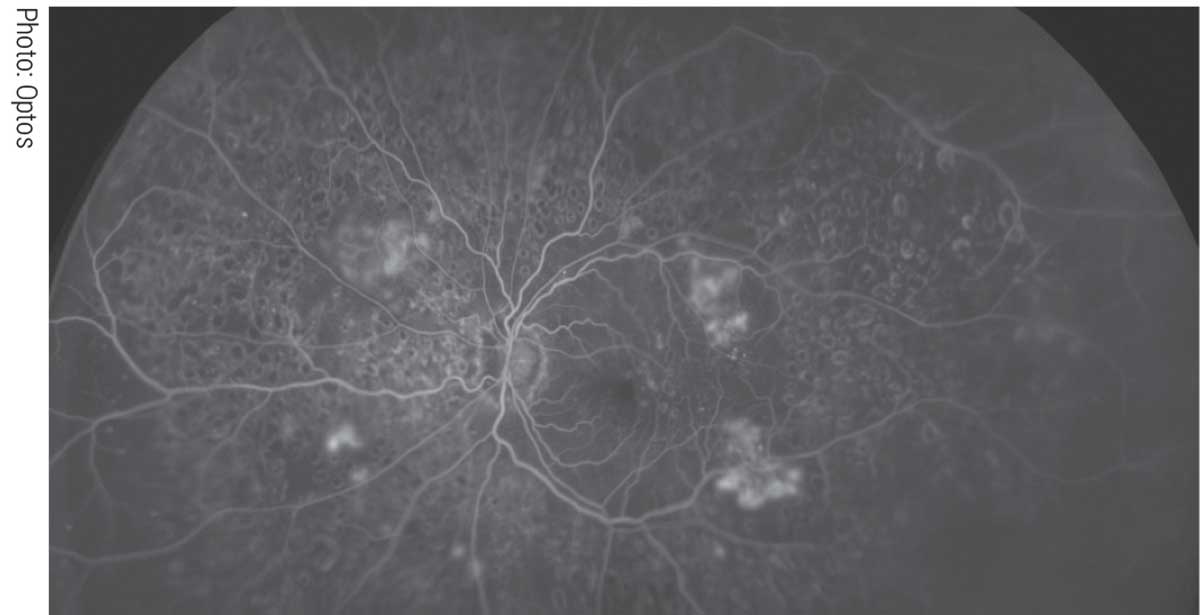 |
| Ultra-widefield imaging’s expansive views of the retina highlight the shortcomings of conventional diabetic retinopathy staging. Click image to enlarge. |
No new staging systems have been validated yet, but as one source recommends, we can adjust our staging in a number of ways. This may not only include peripheral retinal pathology but also a more comprehensive assessment of visual outcomes, as well as better staging of both neovascularization and macular edema. The addition of these measures will need to be evaluated for impact on disease progression and visual outcomes but could provide further clarification and staging of retinopathy for diabetic patients.18
Dr. Seng: I agree that we should be able to ditch this; we should be able to do better. Honestly, standardized photos have not done much to standardize individuals’ clinical staging diagnosis (I often see mild/moderate/severe used inconsistently) or decision-making. However, until there is a better system in widespread use, it is better than nothing.
Blood Pressure and A1c Readings
Dr. Taylor: Hypertension (HTN) and diabetes are systemic diseases that have potentially sight-threatening complications. Are “normal” readings “normal” for everyone? Well, it depends. Knowing what is normal based on age and ethnicity is important, since it can play a vital role in what is considered normal for your particular patient.
Guidelines based on the 2017 results of the Systolic Blood Pressure Intervention Trial have a blood pressure reading of 130/80 as the criteria for the diagnosis of hypertension. No differentiation was made between different age groups with these guidelines, which were endorsed by the American Heart Association and the American College of Cardiology. The study concluded that having a systolic pressure of no more than 120mm Hg reduced the risk of heart attacks, heart failure and stroke over a three-year period. A systolic reading between 130 of 139 or a diastolic reading of 80 to 89 is classified as Stage 1 HTN. Stage 2 HTN occurs when systolic readings are 140 or higher or diastolic readings are over 90.19
Be cognizant of racial disparities in the incidence of HTN, along with patients who have comorbidities such as diabetes, chronic kidney disease and cardiovascular disease. African Americans statistically have higher blood pressure readings and suffer from hypertension at an earlier age than Caucasians. The reasons for racial differences in higher blood pressure and the associated risks are not clearly understood, but with African Americans at dramatically higher risk for stroke and end-stage kidney disease related to HTN, early diagnosis and appropriate referral is essential.20
In diabetes management, the A1c readings help guide management decisions. The A1c test measures the amount of glucose in blood attached to hemoglobin. The result is a percentage, with a normal reading being below 5.7%. There are hemoglobin variants, though, that can affect A1c readings. These variants are inherited from one’s parents and have an ethnicity pattern. Since 2006, all states screen newborns for hemoglobin S, which is associated with sickle cell disease. Some states also screen for hemoglobin C and hemoglobin E disease. Suspect a hemoglobin variant in your patient if they have a family history of blood disorders or have family from a region of the world where variants are common.21
Clinical Monitoring/Management
How we examine the eye, and what we look for, is another moving target. Let’s see how a few staples are holding up today.
Dilated Exams
Dr. Hicks-Hubbard: The annual dilated exam is often dreaded by patients due to side effects and longer wait times in-office; therefore, some providers may feel that UWF photography is a way to combat those concerns. While UWF photography does alleviate those issues, dilation is here to stay. Dilation is still considered the gold standard for assessment and diagnosis of the internal structures of the eye.22
Peripheral retinal pathology is still better visualized when performing a dilated funduscopic exam when compared to UWF photography. One study found that for retinal pathology anterior to the equator, one UWF camera was only sensitive for 45% of pathology detected via dilated fundoscopic exam.22 That being said, another study demonstrated that imaging of ocular tumors can be documented, as well as managed, using UWF photography.23 As mentioned above, UWF photography can also provide additional information in the management of diabetic retinopathy.17 While the frequency of dilation is determined on a case-by-case basis and photography certainly offers some advantages, it seems that these work best in partnership with one another rather than exclusivity.
Dr. Seng: There may come a time when photography may be good enough to replace a dilated fundus exam, but I am doubtful. UWF cameras do allow for a more complete exam when a patient refuses dilation, or cannot be dilated, but they do not provide nearly good enough quality, nor do they get peripheral enough in all quadrants on a consistent basis to replace a properly performed dilated exam. Most patients will agree to the procedure once they are educated on its importance and are prepared and expecting it as part of their complete exam at appropriate frequency as indicated by ocular and medical history.
Bull’s-eye Maculopathy Screening
Dr. Seng: Chloroquine and hydroxychloroquine have long been known to be causative agents for vision-threatening bulls-eye maculopathy. However, the best clinical practices for early detection have evolved over time. Monitoring until the macular pigment changes that make up a bull’s-eye can be detected is long outdated, as this represents end-stage disease with irreparable vision loss. Total dose prescribed (mg/kg of actual weight/day) and length of treatment (cumulative dose) are still important factors (the American Academy of Ophthalmology considers over 1,000 total grams to be at risk), as are other compounding risks such as tamoxifen use or kidney disease. However, the at-risk timeframe can be shorter than previous publications have suggested, and overall incidence of toxicity somewhat higher.24
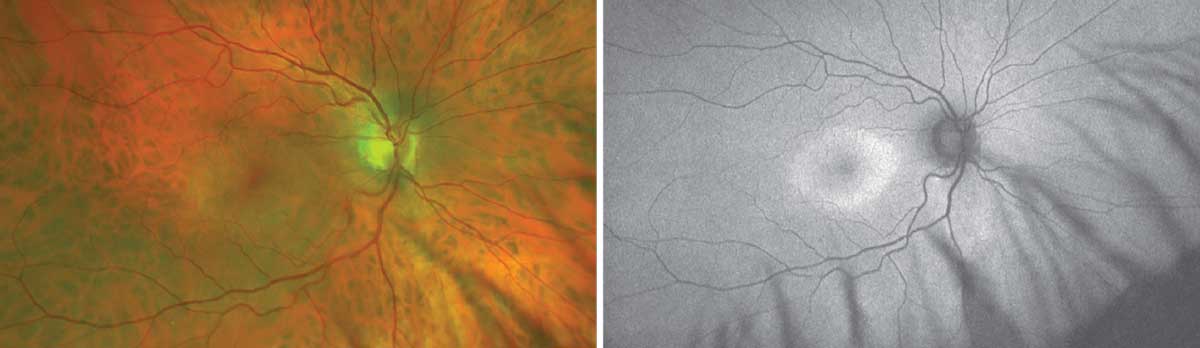 |
| Optos photo showing parafoveal outer retinal and RPE atrophy and FAF imaging showing parafoveal hyper-AF of classic bull’s-eye maculopathy seen in Plaquenil toxicity. Click image to enlarge. |
In-office Amsler grid, color vision testing and fundus photos no longer play a significant role in preventative monitoring, according to the newest guidelines set forth in 2016. The key monitors that currently need to be employed include SD-OCT scans to evaluate for parafoveal photoreceptor ellipsoid zone discontinuity, autofluorescence imaging, 10-2 visual field testing and, when indicated, multifocal electroretinogram.25 Of particular note, however, is that maculopathy in patients of Asian descent can develop outside of the macular zone, so 30-2 or 24-2 visual field testing should be employed, and the zone of monitoring with SD-OCT and autofluorescence should be adjusted to either widefield or off-axis testing.26 We must be sure to follow up-to-date clinical practice guidelines to minimize patient morbidity in relation to this medication.
Dr. Weidmayer: I agree; if we ever see a bull’s-eye, we’ve missed the boat.
Dr. Taylor: I agree.
“Lumpy Bumpy” and “Lazy V” on OCT for Disc Drusen
Dr. Weidmayer: In the early years of OCT, we latched onto how a “lumpy-bumpy” internal contour of the optic disc indicated optic disc drusen, differentiating it from disc edema where we saw a “lazy V” pattern with a smooth contour.27 The “lumpy-bumpy” vs. “lazy V” criteria gave us just less than two-thirds for both sensitivity and specificity; nonetheless, at the time this was tremendously helpful.27
However, with a shift from time-domain to spectral and swept-source OCT, we can now physically see optic disc drusen on OCT, and we also have tools such as autofluorescence imaging to easily highlight disc drusen. When all else fails we still have B-scan, which sorts this out nicely. “Lumpy-bumpy” vs. “lazy V” for optic disc drusen may be obsolete terminology.
Dr. Taylor: Optic nerve drusen are a good excuse to have a B-scan!
Dr. Weidmayer: Preach it, Brad.
IOP Adjustment Factors
Dr. Weidmayer: Using a historic IOP adjustment factor for applanation tonometry to determine a corrected IOP depending on central corneal thickness (CCT) was never a true clinical calculation—it was a concept.
We do still need to consider how thin and thick CCT can contribute to inaccurate characterization of actual IOP, and we know thin CCT is important in considering glaucoma risk. However, we’ve also learned that corneal biomechanical properties (such as hysteresis) other than just the physical and geometric properties (such as CCT) affect IOP measurements as well, and moreover affect how the optic nerve is able to tolerate the IOP.28 So, IOP is more than just applanation with an adjustment to account for CCT.
Rather than actually adjusting IOP measurements per the old tables, we can instead clinically consider a “thick or thin” mentality for CCT and consider how that—along with myriad other considerations—impacts glaucoma development and progression risk. Save yourself the math and throw away your IOP adjustment table.
Dr. Hicks-Hubbard: I agree with Sara! It is time to ditch the tables. The thickness of the cornea definitely needs to be considered, but a mindset of thick or thin is more relevant rather than an exact conversion.
Dr. Taylor: I agree!
Scratching the Surface
Despite devoting nearly 5,000 words to the subject of diagnostic protocols, it’s clear that the topic could easily merit twice as much coverage—and still not achieve 100% consensus or touch on all the debates in play at any given moment. Such is the fun, and the frustration, of practicing optometry in an environment marked by constant advancement in our knowledge, capabilities and scope. Please share with us your own stance on the above points or others not addressed here!
The views expressed by the authors do not necessarily reflect the positions of the US government or Department of Veterans Affairs.
Dr. Hicks-Hubbard provides ocular telehealth care to veterans throughout Michigan, Indiana and Ohio via the Louis Stokes VA based in Cleveland, OH. She has served as adjunct faculty for several optometry schools across the country.
Dr. Taylor is the supervisory optometrist at the Aleda E. Lutz VA Medical Center in Saginaw, MI, and is a member of the Michigan State Board of Optometry.
Dr. Weidmayer practices at the LTC Charles S. Kettles Medical Center, VA Ann Arbor Healthcare System in Ann Arbor, MI. She is also a clinical assistant professor for the Department of Ophthalmology and Visual Sciences, WK Kellogg Eye Center of the University of Michigan.
Dr. Seng practices at the LTC Charles S. Kettles Medical Center, VA Ann Arbor Healthcare System. She is also a clinical instructor for the Department of Ophthalmology and Visual Sciences, WK Kellogg Eye Center of the University of Michigan and a clinical associate professor at Michigan College of Optometry of Ferris State University.
They have no financial disclosures.
|
1. Trevino R, Kynn MG. Macular function surveillance revisited. Rev Optom. 2008;79(7):397-403. 2. Kampmeier J, Zorn MM, Lang GK, et al. Comparison of the preferential hyperacuity perimeter (PHP) test and amsler grid test in the diagnosis of different stages of age-related macular degeneration. Klin Monbl Augenheilkd. 2006;223(9):752-6. 3. National Research Council (US) Committee on Vision. Procedures for Testing Color Vision: Report of Working Group 41. Washington (DC): National Academies Press (US); 1981. PMID: 25032450. 4. Shahinfar S, Johnson LN, Madsen RW. Confrontation visual field loss as a function of decibel sensitivity loss on automated static perimetry. Implications on the accuracy of confrontation visual field testing. Ophthalmology. 1995;102(6):872-7. 5. Fayed AE, Fawzi A. OCTA vs dye: the pros and cons. Rev Ophthalmol. www.reviewofophthalmology.com/article/octa-vs-dye-the-pros-and-cons. January 5, 2019. Accessed January 25, 2022. 6. Maslin JS, Barkana Y, Dorairaj SK. Anterior segment imaging in glaucoma: an updated review. Indian J Ophthalmol. 2015;63(8):630-40. 7. Sakata LM, Lavanya R, Friedman DS, et al. Comparison of gonioscopy and anterior segment ocular coherence tomography in detecting angle closure in different quadrants of the anterior chamber angle. Ophthalmology. 2008;115(5):769-74. 8. Xu BY, Pardeshi AA, Burkemper B, et al. Differences in anterior chamber angle assessments between gonioscopy, EyeCam and anterior segment OCT: the Chinese American Eye Study. Transl Vis Sci Technol. 2019;8(2):5. 9. Thomas C. Clinical: the back. Optometric Management. November 2014;49:34-5, 45. 10. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15(3):276-83. 11. Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15(3):539-74. 12. Scott CA, Catania LJ, Larkin KM, et al. Care of the patient with ocular surface disorders. American Optometric Association. www.my.ico.edu/file/CPG-10---Ocular-Surface-Disorders.pdf. 13. Early Treatment Diabetic Retinopathy Study Research Group. Grading Diabetic Retinopathy from Stereoscopic Color Fundus Photographs-an Extension of the Modified Airlie House Classification. ETDRS Report Number 10. Ophthalmology. 1991;98(5):786-806. 14. Solomon SD, Goldberg MF. ETDRS grading of diabetic retinopathy: still the gold standard? Ophthalmic Res. 2019;62(4):190-5. 15. Zafar S, Smith K, Boland MV, et al. Real-world outcomes among eyes with center-involving diabetic macular edema and good visual acuity. Curr Eye Res. 2020;45(7):879-87. 16. Baker CW, Glassman AR, Beaulieu WT, et al. Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: a randomized clinical trial. JAMA. 2019;321(19):1880-94. 17. Aiello LP, Odia I, Glassman AR, et al. Comparison of early treatment diabetic retinopathy study standard 7-field imaging with ultrawide-field imaging for determining severity of diabetic retinopathy. JAMA Ophthalmol. 2019;137(1):65-73. 18. Sun JK, Aiello LP, Abràmoff MD, et al. Updating the staging system for diabetic retinal disease. Ophthalmology. 2021;128(4):490-3. 19. Reading the new blood pressure guidelines. Harvard Health Publishing. www.health.harvard.edu/heart-health/reading-the-new-blood-pressure-guidelines. November 16, 2021. Accessed January 25, 2022. 20. Lackland DT. Racial difference in hypertension: implications for high blood pressure management. Am J Med Sci. 2014;348(2);135. 21. Association of Public Health Laboratories and Centers for Disease Control and Prevention. Hemoglobinopathies: current practices for screening, confirmation and follow-up. Silver Spring, MD: Association of Public Health Laboratories; 2015. www.cdc.gov/ncbddd/sicklecell/documents/nbs_hemoglobinopathy-testing_122015.pdf 22. Mackenzie PJ, Russell M, Ma PE, et al. Sensitivity and specificity of the optos optomap for detecting peripheral retinal lesions. Retina. 2007;27(8):1119-24. 23. Callaway NF, Mruthyunjaya P. Widefield imaging of retinal and choroidal tumors. Int J Retina Vitreous. 2019;5(Suppl 1):49. 24. Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132:1453-60. 25. Marmor MF, Kellner U, Lai TY, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2016;123:1386-94. 26. Melles RB, Marmor MF. Pericentral retinopathy and racial differences in hydroxychloroquine toxicity. Ophthalmology. 2015;122:110-6. 27. Johnson LN, Diehl ML, Hamm CW, et al. Differentiating optic disc edema from optic nerve head drusen on optical coherence tomography. Arch Ophthalmol. 2009;127(1):45-9. 28. Belovay GW, Goldberg I. The thick and thin of the central corneal thickness in glaucoma. Eye (Lond). 2018;32(5):915-23. |