And then there were two… targeted drugs for presbyopia, that is. Soon to join Allergan’s Vuity (pilocarpine 1.25%) on the market is another pilocarpine drop, this one at a lower concentration of 0.4%. Called Qlosi (pronounced “CLOH-see”), the drug's FDA approval was announced earlier today by Orasis Pharmaceuticals. The new drop is approved for daily or BID dosing as needed for patients with presbyopia. Given Qlosi’s lower concentration, clinicians will be curious to see whether it results in fewer adverse effects than Vuity. Another potential plus: the formulation is preservative-free, Orasis says.
 |
| The second approved drop for presbyopia, called Qlosi, has a lower pilocarpine concentration than Vuity (0.4% vs. 1.25%). Click image to enlarge. |
In the drug’s two Phase III clinical trials, involving more than 600 patients, Orasis reports that the pupil-constricting drop demonstrated efficacy 20 minutes after administration and—with the benefit of a second dose two to three hours after the first—can last up to eight hours, as measured on day 15 (see chart). Both trials also met their primary and key secondary endpoints on day eight, achieving a gain of at least three lines in distance-corrected near visual acuity without losing one line or more in distance visual acuity. The company adds in its press release that the drop had no impact on night vision.
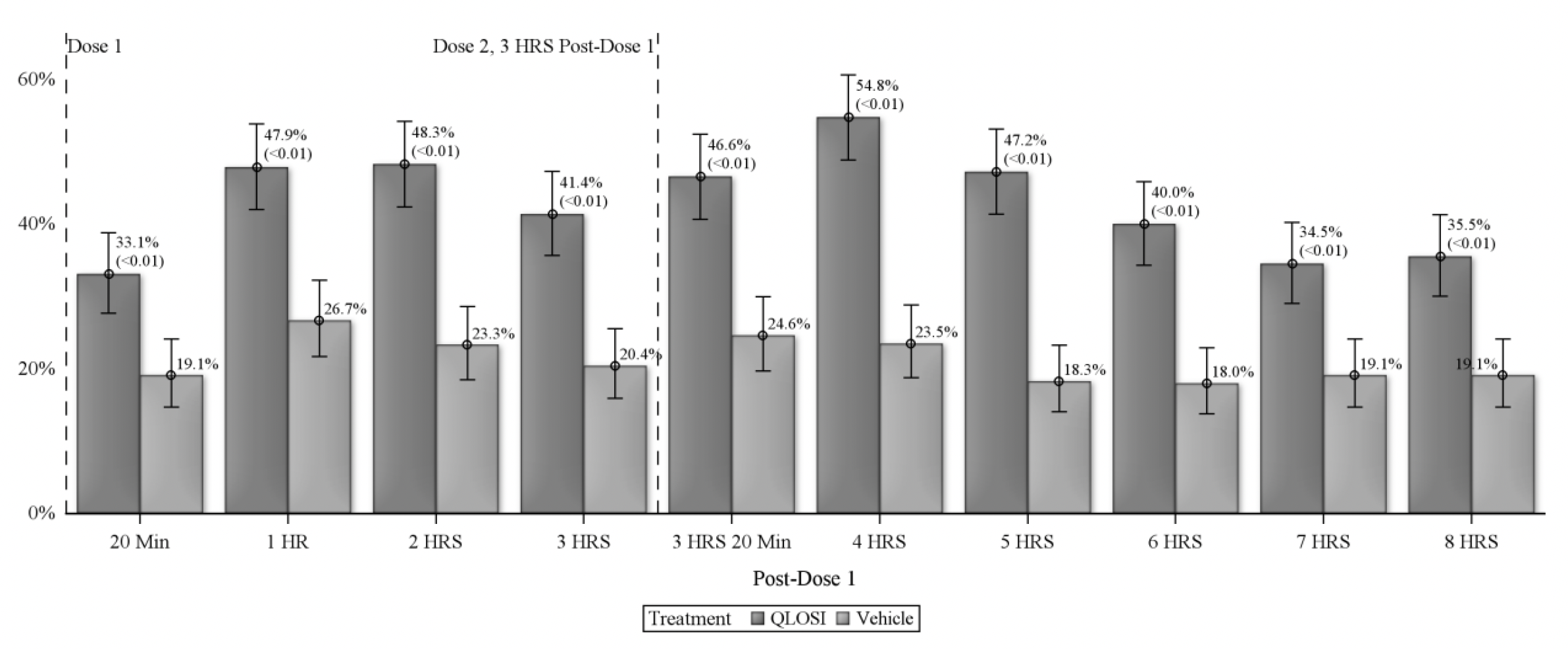 |
| Treatment efficacy was noted as early as 20 minutes after the first dose, with one-third of subjects (33.1%) meeting the desired endpoint at that time. Response rate increased at both the one-hour (47.9%) and two-hour (48.3%) marks, then dropped a small amount. Following a second drop administration three hours after the first, the maximum response rate (54.8% of subjects) was seen at hour four. By the eight-hour mark, just over one-third of subjects (35.5%) still maintained the appropriate near vision gains to meet the definition of treatment response. Click image to enlarge. |
Headache and instillation site pain were among the most common treatment-related adverse events, affecting 6.8% and 5.8% of participants, respectively. Moderate treatment-related adverse events were reported by 1.3% of participants, and all other adverse events were mild, the company reports.
Optometrists and their patients can expect the drug to be commercially available in the first half of 2024, Orasis says.
For more information, go to www.orasis-pharma.com.

