A Closer Look at EyelidsThe eyelids are the first structure examined under the slit lamp microscope and are often quickly evaluated. Yet accurate assessments of common eyelid conditions and appropriate medical management can improve patients' quality of life. The October issue of Review of Optometry gives readers a rundown on best practices regarding eyelid health. |
Demodex blepharitis—a prevalent yet underdiagnosed and undertreated condition—affects at least 25 million Americans and can have a significant impact on an individual’s daily life.1 Characterized by collarettes, which lead to a number of associated symptoms such as red, irritated and itchy eyelids, it accounts for more than two-thirds of all blepharitis cases.1,2
Until recently, management options for Demodex blepharitis have been limited. Clinicians have traditionally relied on encouraging patients to practice better lid hygiene to reduce bacterial overgrowth and recommending eyelid cleansers with tea tree oil to help eradicate mites. However, this past July, the long-awaited first targeted therapeutic for the condition finally received FDA approval. Formerly known as TP-03 during its development stage, Xdemvy (lotilaner ophthalmic solution 0.25%, Tarsus Pharmaceuticals) requires a six-week course of treatment and involves one drop of the solution in each eye, administered twice daily approximately 12 hours apart.
The approval of Xdemvy was supported by findings from two randomized, vehicle-controlled studies—known as Saturn-1 and Saturn-2—involving 833 patients who received either Xdemvy or vehicle. Both trials met the primary endpoint of collarette reduction to no more than two per upper eyelid by day 43 as well as the secondary endpoints of mite eradication (zero per lash) and erythema cure (grade zero). The safety profile of Xdemvy was also favorable in both studies.
In Saturn-1, 81% of patients receiving treatment had a collarette grade of zero or one on day 43 vs. 23% for placebo. In Saturn-2, these percentages were 89% vs. 33%, respectively. The Saturn-2 study authors additionally reported that treatment led to a clinically meaningful collarette reduction to 10 collarettes or fewer (89.1%), mite eradication (51.8%), erythema cure (31.1%) and composite cure (19.2%).3
In many cases, the treatment also showed effectiveness in as little as two weeks; by day 15 in Saturn-1, 68% of patients achieved complete mite eradication vs. 18% of those on placebo.
As one of the study authors and a clinical investigator for Saturn-1 and Saturn-2, Paul Karpecki, OD, director of Cornea and External Disease for Kentucky Eye Institute and chief clinical editor of this magazine, has had firsthand experience with Xdemvy and believes its recent FDA approval will have a significant impact on patients and optometric practice.
“About 58% of all patients entering an optometry practice have collarettes, the pathognomonic sign for Demodex blepharitis,” he notes. “We have never had a prescription drop for this most common cause of blepharitis until Xdemvy. Given the data on eradication rates, this will be a welcome therapeutic.”
Though Elyse Chaglasian, OD, associate dean for faculty at the Illinois College of Optometry, has not yet had the opportunity to experience this new agent firsthand, she believes Xdemvy could benefit a variety of patients, and she looks forward to incorporating it into clinical practice. “It is gratifying to see years of research come to fruition,” she says. “This new treatment fills a gap in care and has the potential to significantly improve the quality of life for our patients with Demodex blepharitis.”
Below, you’ll find answers to five common questions clinicians may be asking about this new therapeutic agent, helping you better understand its clinical role and how to optimize its use in your practice.
1. Where does this new drug fit into clinical practice?
Now that Xdemvy is available, optometrists must determine when it should be prescribed and who the best candidates are for this approach. In Dr. Karpecki’s opinion, any patient with more than two collarettes should consider treatment.
“My experience is that Demodex builds over time, eventually leading to scalloped lid margins, meibomian gland dysfunction (MGD), thin or missing lashes, atrophy of the meibomian glands and chronic evaporative dry eye, to name a few consequences,” he notes.
“I would want this ectoparasite eradicated as soon as it’s identified as opposed to waiting for significant symptoms such as irritation, itching, recurrent hordeola or chalazia,” he explains. “I would also recommend gently rubbing the base of the lashes with any excess drop after instillation in the eyes BID.”
Diagnosing a patient with Demodex blepharitis does not require additional tools or equipment, according to Dr. Karpecki, who advises clinicians to “look for collarettes by having the patient look down while at the slit lamp, scanning the lid margins with slightly higher magnification.”
Dr. Chaglasian is already considering which of her patients might be candidates for Xdemvy. “I treat a woman who is your typical dry eye patient with multiple issues, including MGD and Demodex. Her main complaint is red and irritated eyelids,” she says. Dr. Chaglasian adds that while treatments, including low-level light therapy and tea tree and okra-based lid wipes, have helped this patient, they have not resolved the issue completely.
“She is, in my opinion, the perfect candidate. We have exhausted available options and Xdemvy will hopefully provide the relief she needs,” Dr. Chaglasian notes. “This isn’t just another artificial tear or lid wipe. Xdemvy is a class of drug that we have never had before in Demodex blepharitis that can treat a condition that is endemic among our patients.”
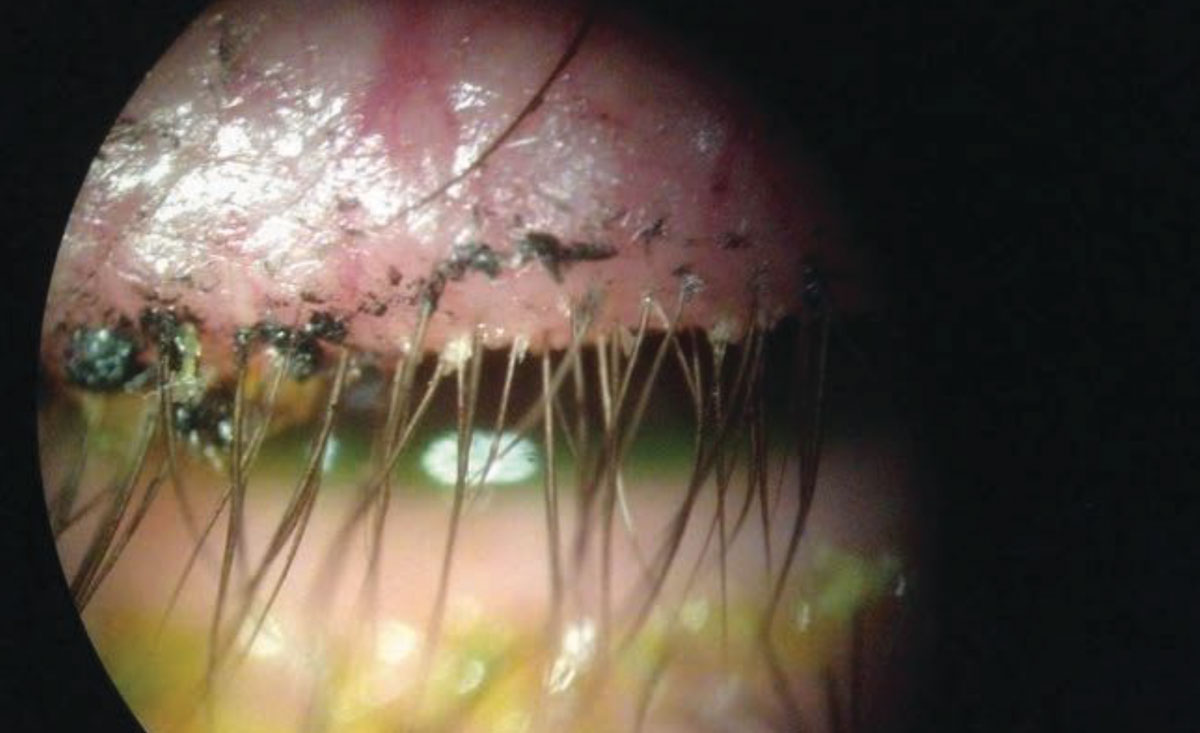 |
|
This patient’s lid margins display Demodex infestation at the base of the lashes. Click image to enlarge. |
2. Should other treatments be used simultaneously?
Coupling Xdemvy with other treatments (e.g., meibomian gland expression, warm compresses, lid scrubs, blepharoexfoliation) could prove beneficial in some cases. Depending on the specific needs of your patient, a multifaceted approach may offer the best outcomes.
“I do believe therapeutics do best when surrounded by other treatment options,” notes Dr. Karpecki. For example, he suggests that a case of moderate to severe Demodex blepharitis would benefit from blepharoexfoliation to remove the bulk of pathology and collarettes, combined with Xdemvy for eradication of the mites.
“Another option could be a low-level light therapy blue mask with intense pulsed light (IPL) treatment followed by Xdemvy for six weeks,” he says. “Or, one could simply prescribe Xdemvy BID for six weeks and then begin a manuka extract hygiene product such as MyboClean (Danelli Ocular Creation) once daily long-term to maintain lid hygiene.”
Given the high association between MGD and Demodex blepharitis, Dr. Karpecki would recommend warm hydrating compresses for these patients.
When discussing the use of ivermectin, he notes, “since oral ivermectin requires Kg conversion to determine dosage, I am hopeful that Xdemvy, with its high eradication rates, will remove the need for this oral medication going forward except for use in extreme cases.”
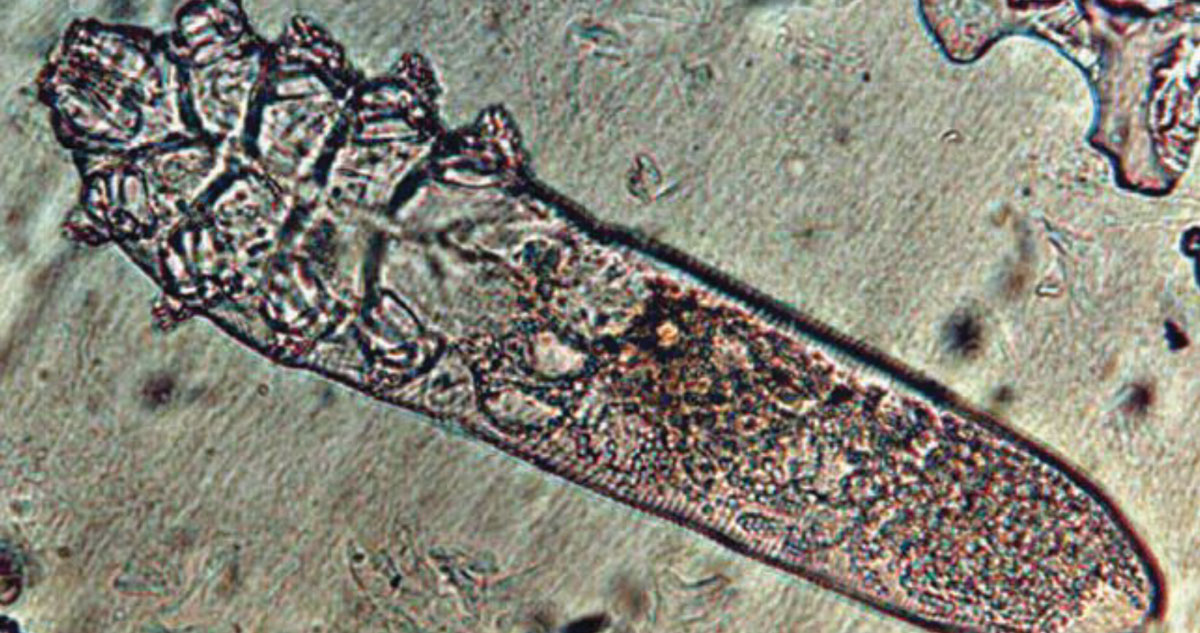 |
This image depicts a Demodex folliculorum mite under a microscope. While these critters can be found on the skin of most humans, an overpopulation may lead to ocular surface discomfort and require intervention. Click image to enlarge. |
3. What if patients don't achieve good enough results from Xdemvy alone?
The majority of patients who received Xdemvy in Saturn-1 and Saturn-2 clinical trials benefited from treatment. As previously noted, more than 50% of patients in Saturn-2 achieved complete collarette cure—defined as zero to two collarettes per lid at day 43—and 89% had a significant, clinically meaningful collarette cure.3
Saturn-1 data showed that a greater proportion of patients in the study group achieved a clinically meaningful collarette cure (81.3% vs. 23.0%), complete collarette cure (44.0% vs. 7.4%), mite eradication (67.9% vs. 17.6%), erythema cure (19.1% vs. 6.9%) and composite cure (13.9% vs. 1.0%) when compared with the control group.4
While these results are promising and suggest that most patients will benefit from Xdemvy, optometrists must be prepared to care for patients who don’t see the full benefit of this agent after the six-week treatment period. So, what is the contingency plan in these cases?
When asked about the best approach for patients who don’t respond to treatment, or have a suboptimal outcome, Dr. Karpecki recommends considering IPL in addition to blue-light low-level light therapy with blepharoexfoliation. However, he notes that based on the positive trial results, “my expectation is that there will be very few patients that don’t experience a significant improvement after six weeks of Xdemvy.”
One possibility to consider in more severe or persistent cases of Demodex blepharitis is whether the patient may be experiencing comorbid Demodex overgrowth on their facial skin that is preventing the condition’s full resolution. See the sidebar on the following page to learn more about how these patients may potentially benefit from pairing targeted lid therapies with dermatologic treatment.
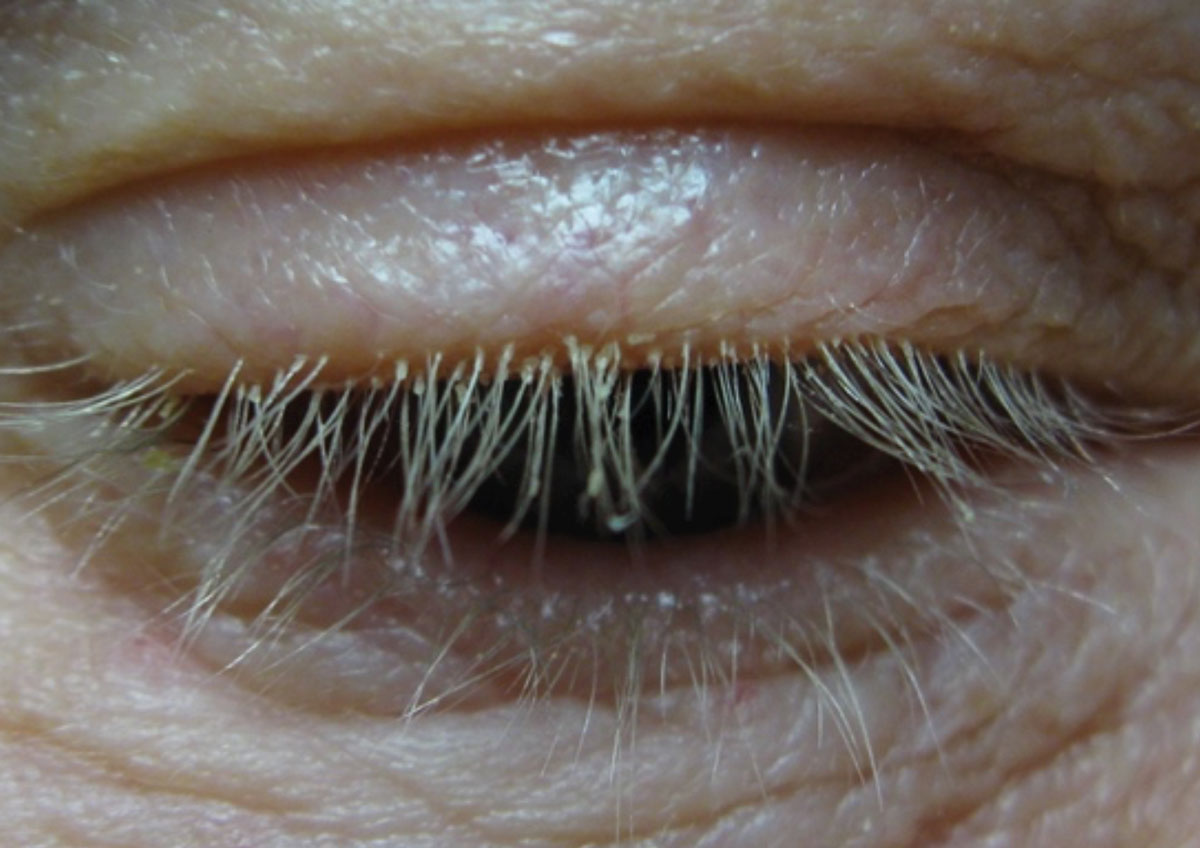 |
Cylindrical dandruff that presents at the base of the lashes, shown here, is a telltale sign of Demodex infestation. Click here to enlarge image. |
4. How should I address any adverse effects that arise from treatment?
Like any therapeutic agent, Xdemvy is not immune to potential side effects. Therefore, optometrists must be prepared to not only address any adverse events that occur but also educate patients prior to prescribing the drug.
According to the prescribing information, the most common ocular adverse reactions in the clinical trials were instillation site stinging and burning, which was observed in 10% of patients. More severe adverse effects, mainly chalazion/hordeolum and punctate keratitis, were reported in less than 2% of patients treated with Xdemvy.2
In Saturn-1, 19.8% of patients who received Xdemvy had at least one treatment-emergent adverse event compared with 21.5% in the control group. The study authors reported that all treatment-emergent adverse events were mild.4
The most common adverse event reported among patients in the Xdemvy cohort in Saturn-1 was instillation site pain (11.8%). Other, less frequent ocular treatment-emergent adverse events observed in the study and control groups included instillation site pruritus (1.4% vs. 3.3%), reduced visual acuity (2.8% vs. 2.9%), eye pain (1.4% vs. 1.4%), eye discharge (1.4% vs. 1.0%) and chalazion (0.5% vs. 1.4%), all of which were reported to be mild.4
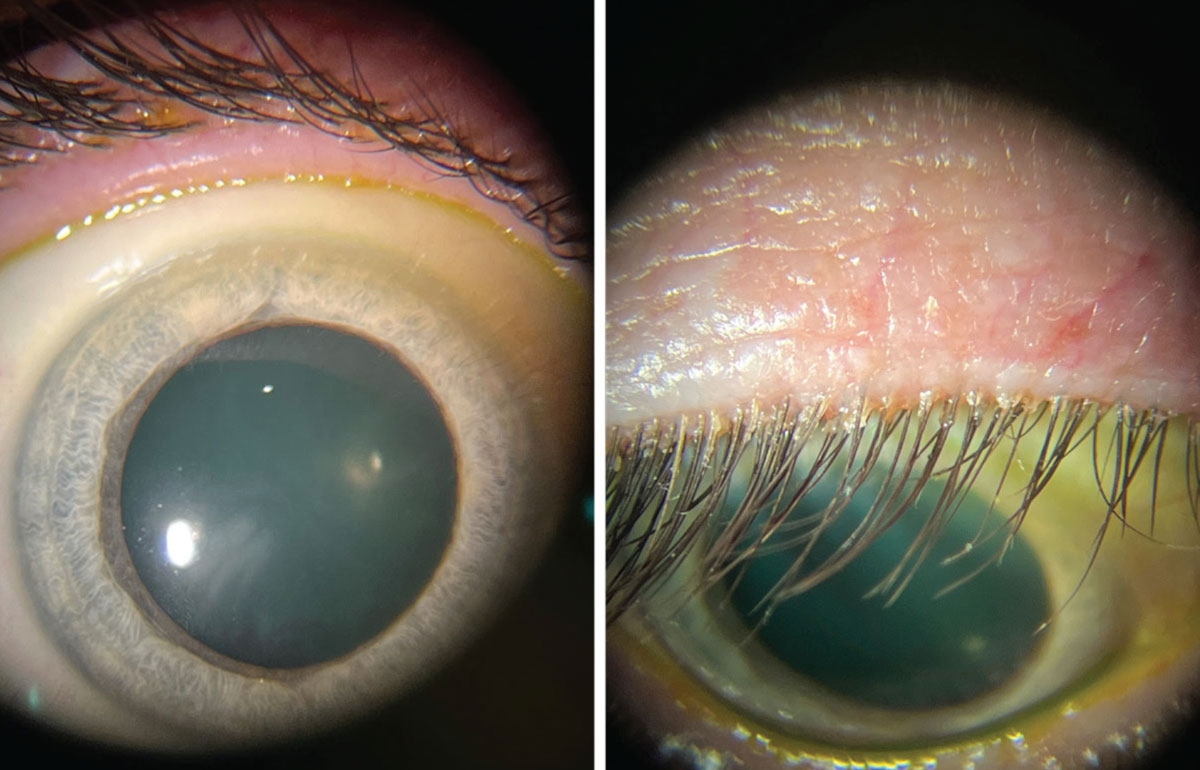 |
|
These photos show the same patient during a slit lamp exam. Left: Patient looking straight ahead with minimal identifiable blepharitis. Right: Patient looking down with obvious collarettes, indicating a Demodex infestations. Click image to enlarge. |
The most common adverse event reported among patients in the Xdemvy cohort in Saturn-1 was instillation site pain (11.8%). Other, less frequent ocular treatment-emergent adverse events observed in the study and control groups included instillation site pruritus (1.4% vs. 3.3%), reduced visual acuity (2.8% vs. 2.9%), eye pain (1.4% vs. 1.4%), eye discharge (1.4% vs. 1.0%) and chalazion (0.5% vs. 1.4%), all of which were reported to be mild.4
Safety findings from Saturn-2 were consistent with Saturn-1, further demonstrating the safety and tolerability of Xdemvy among patients with Demodex blepharitis. No serious treatment-related adverse events were observed. The study authors reported that in Saturn-2, instillation site pain, burning and/or stinging was experienced by 16 (7.9%) patients, and three patients (1.5%) developed signs or symptoms of dry eye. Additionally, 91% of patients found the drop to be neutral to very comfortable.3
Despite the strong safety profile reported in clinical trials, optometrists must clearly outline any potential side effects to patients and readily offer support to those who experience them.
“It is important to set expectations for patients even if 90% may not develop adverse effects,” advises Dr. Karpecki. “I would let patients know that they may experience burning or stinging upon instillation of the drop and that it’s normal,” he says. “There were very few patients in the clinical trials that discontinued use due to adverse events like instillation site stinging and burning.”
No one wants a surprise adverse reaction, emphasizes Dr. Chaglasian. “It all comes down to patient education,” she notes. “Optometrists need to take the time to not only communicate the benefits but also any possible side effects, no matter how unlikely.”
5. How do I navigate insurance coverage?
“Insurance providers all wait six months before adding a new therapy to a formulary, so companies have to find alternate ways to make their drug available to patients,” he notes. “Specialty pharmacies, such as BlinkRx, make the drug more easily available and for discounted rates—typically $50 or less.”
Dr. Karpecki says that he believes this price is reasonable, especially when you consider that the drug is not a long-term therapy like many immunomodulators used in, for example, dry eye disease. Other specialty pharmacy options where you might be able to order Xdemvy for a lower price include AllianceRx Walgreens Pharmacy, Carepoint Pharmacy and CVS Specialty Pharmacy.
When dealing with insurance coverage for Xdemvy, “be sure to have the correct ICD-10 diagnosis codes, which should include both H01.00 (unspecified blepharitis) and B88.0 (other acariasis),” Dr. Karpecki adds.
 |
Takeaways
The introduction of Xdemvy into clinical practice addresses a huge unmet need for patients with Demodex blepharitis, Dr. Chaglasian says. “We have in-office treatments that can be effective for a short time, but at-home treatments, such as lid wipes, offer mixed results at best and don’t take care of the underlying problem,” she explains.
“Xdemvy has the potential to provide long-term relief for our patients,” Dr. Chaglasian reiterates. “For so long, we have been doing the best we can with what we have, but now we have a product that fills this gap. It is a game changer for the treatment of this prevalent condition.”
1. Karpecki P. Call the exterminator. Review of Optometry. 2023;160(9):84. 2. Tarsus announces positive topline data from Saturn-2 phase 3, the second pivotal trial of TP-03 for the treatment of Demodex blepharitis, and expects to file a new drug application this year. Tarsus Pharmaceuticals. Published May 02, 2022. ir.tarsusrx.com/news-releases/news-release-details/tarsus-announces-positive-topline-data-saturn-2-phase-3-second. Accessed September 18, 2023. 3. Gaddie IB, Donnenfeld ED, Karpecki P, et al. Lotilaner ophthalmic solution 0.25% for Demodex blepharitis: randomized, vehicle-controlled, multicenter, phase 3 trial (Saturn-2). Ophthalmology. June 5, 2023. [Epub ahead of print]. 4. Yeu E, Wirta DL, Karpecki P, et al. Lotilaner ophthalmic solution, 0.25%, for the treatment of Demodex blepharitis: results of a prospective, randomized, vehicle-controlled, double-masked, pivotal trial (Saturn-1). Cornea. 2023;42(4):435-43. |

