A Closer Look at EyelidsThe eyelids are the first structure examined under the slit lamp microscope and are often quickly evaluated. Yet accurate assessments of common eyelid conditions and appropriate medical management can improve patients' quality of life. The October issue of Review of Optometry gives readers a rundown on best practices regarding eyelid health. |
Ptosis typically refers only to drooping of the upper eyelid, with drooping of the lower eyelid termed reverse ptosis. There are two muscles that assist in the elevation of the eyelid: the levator palpebrae superioris (LPS) and the superior tarsal muscle, also known as Muller’s muscle (MM). When these muscles are not functioning properly, it can result in a droopy, or ptotic eyelid. The primary muscle responsible for elevation is the LPS, which when damage occurs, results in a more prominent ptosis. In contrast, when the MM is damaged, it results in a more subtle ptosis. There are four categories of ptosis: aponeurotic, myogenic, neurogenic and mechanical. Through a thorough case history and examination, eyecare providers can differentiate between these categories and etiologies of the condition.
History Questions
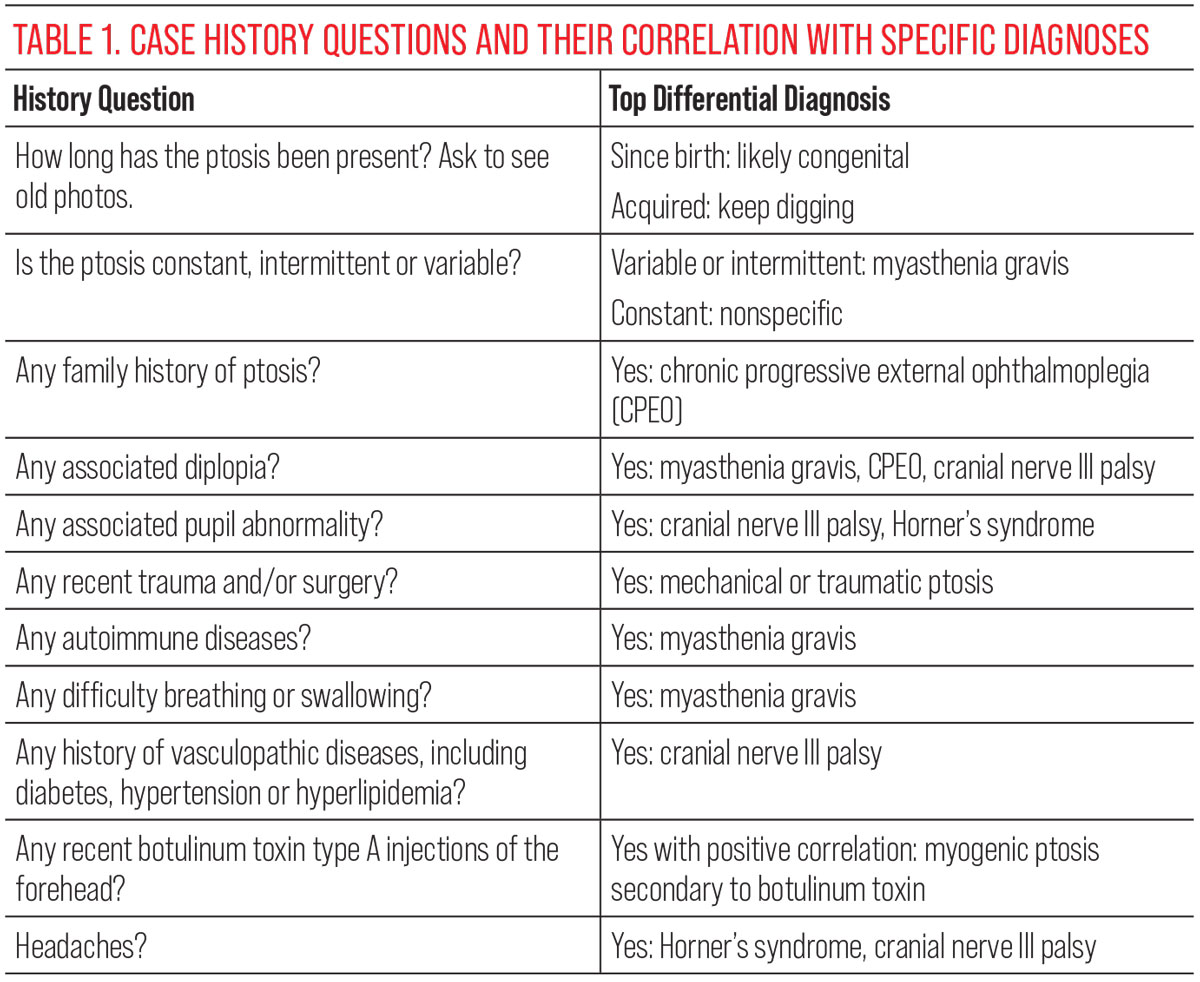
Case history is an important tool for eyecare providers to differentiate between various types and etiologies of ptosis (Table 1). First, ask the patient if they have noticed any change in the appearance of their eyelids, and if so, when it was first noted. If they cannot give a specific timeline, old photos can be used to determine the longevity of the ptosis. Ask the patient if there is any family history of ptosis or other eye conditions and inquire if any specific event that may have resulted in ptosis has occurred, such as any ocular trauma, surgery, contact lens use or botulinum toxin type A injections in and around the forehead/ocular region.1,2 The next step is to investigate the variability of the ptosis. Ask if there is a time of day when the ptosis is more noticeable or if worse when tired.
 |
| Click image to enlarge. |
There are several diagnoses where ptosis is a main clinical finding. Conditions such as myasthenia gravis (MG), cranial nerve III (CN III) palsies, chronic progressive external ophthalmoplegia (CPEO) and Horner’s syndrome (HS) all may present with ptosis. Given the possible etiologies, clinicians should inquire about other ocular complaints, including diplopia and anisocoria. Difficulty breathing or swallowing, headaches and neck pain should also be investigated. Due to possible systemically associated conditions, ask about history of autoimmune diseases, vasculopathic diseases (diabetes, hypertension, hyperlipidemia) and other medical conditions.1
Examination Elements
A thorough eye exam in conjunction with a detailed case history can help pinpoint a working diagnosis. While asking case history questions, take note of the patient’s appearance. Observe any asymmetry of their eyelids or facial structure. Patients may not recognize a subtle eyelid droop; eyecare providers may be the first to notice the ptosis. It is necessary to determine if the abnormal presentation is the result of an enlarged vs. a reduced palpebral aperture. Assess for scars, abnormal blinking, gross lid lesions and eyelid edema, which may point to clues regarding eyelid symmetry.
Additional examination elements can also be quite helpful when assessing for eyelid asymmetry. Exophthalmometry should be conducted to evaluate for pseudoptosis, which is a test measuring how the globe is sitting in the orbit. Both proptosis and enophthalmos may present with asymmetric palpebral apertures and facial appearance.3 Careful extraocular muscle (EOM) motilities should be performed with thorough cover testing in multiple positions of gaze. Variability may be uncovered with multiple cover tests and EOM motility evaluations. Measuring pupil sizes in bright and dim illumination is also helpful in differentiating between the common causes of ptosis.
Patients with true ptosis rather than pseudoptosis may engage their frontalis muscles and tilt their head back to compensate for eyelid droop.3 Eyelid measurements are most accurately obtained when the elevator muscles are isolated. Isolation is achieved by placing a hand on the forehead, neutralizing the frontalis muscle. Palpebral apertures, lid crease, margin-reflex distance and levator function measurements should be performed on every patient with ptosis (Figure 1).
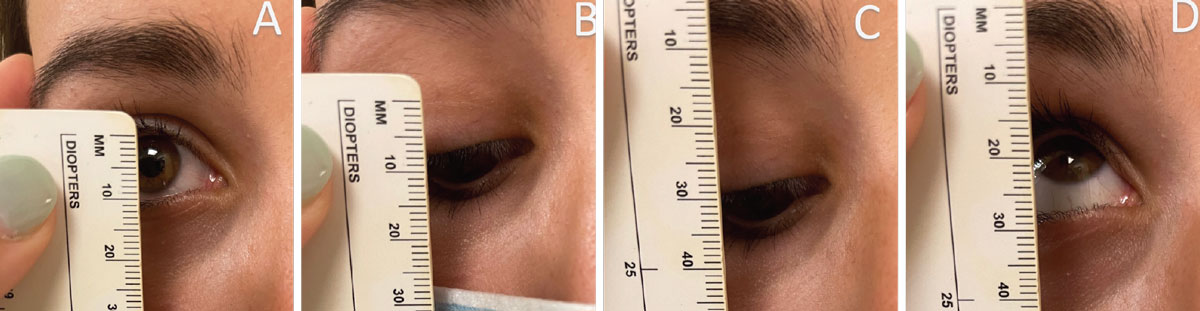 |
Fig. 1. Image A represents measuring palpebral aperture. In this instance, the palpebral aperture is 10mm. Image B represents measuring lid crease; there may be more than one lid crease present and all should be measured. The lid crease here is 7mm. Images C and D represent measuring levator function. Levator function is measured by the difference between down and up gazes. Here, the levator function is 14mm. Click image to enlarge. |
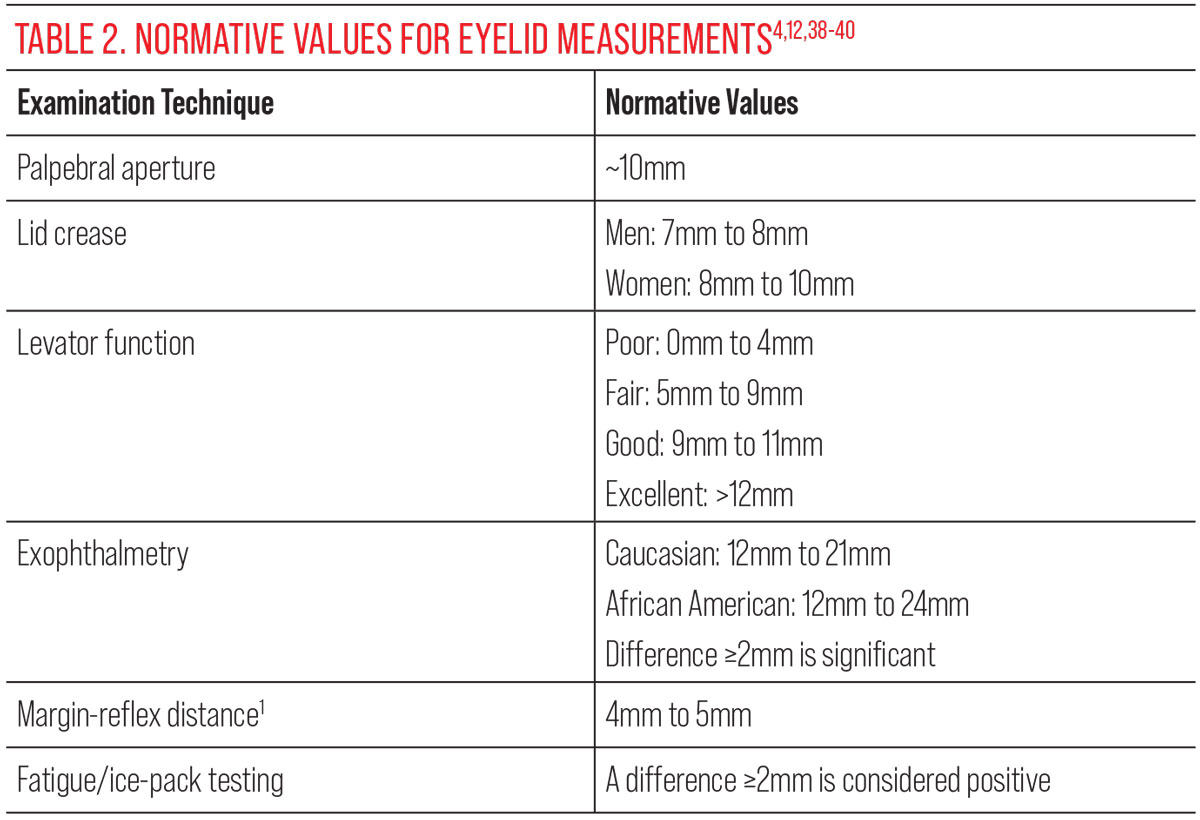
Margin-reflex distance 1 is performed with the patient looking at a transilluminator while the doctor measures the distance between the upper lid margin and corneal reflex.4 Additional eyelid evaluations can be performed, such as fatigue/ice-pack testing, orbicularis oculi strength and assessing for a curtain sign (Table 2). A positive curtain is evidenced by additional drooping of the fellow eyelid when manually lifting the more ptotic eyelid.4 After testing the function of eyelids, a careful ocular health examination should also be performed on them.
 |
| Click image to enlarge. |
Isolated Ptosis
There are many different reasons why ptosis manifests in a patient. The types may require distinct subsequent follow-up care or management, thus it is important to differentiate between the classifications.
Aponeurotic ptosis. This type typically presents in the fifth to sixth decade of life and is the most common type seen in adults.3 Aponeurotic type ptosis is secondary to dehiscence or disinsertion of the levator aponeurosis and is commonly associated with aging. Patients may notice a subtle change in their eyelid appearance over time. While this condition is typically bilateral, it can present asymmetrically. When assessing eyelid measurements, a normal levator function and a high lid crease are common. These patients are not likely to present with abnormal extraocular motilities or pupillary abnormalities.3
Traumatic ptosis. Broadly spanned, traumatic ptosis may be categorized as aponeurotic, myogenic, neurogenic or mechanical, depending on the mechanism of injury. This type is the second most common etiology and can happen when there is damage to the belly of the LPS or the MM or from disinsertion of the levator aponeurosis of the tarsal plate.5 Like aponeurotic ptosis, these patients may present with a high lid crease, and there is typically a cause-and-effect relationship for this etiology. Therefore, case history is vital for diagnosis. Inquiring about any recent trauma to the head or orbital region can help identify this etiology. This type can also be a post-surgical finding related to ocular speculum use in cataract surgery.6
Mechanical ptosis. This can occur secondary to external etiologies that prevent the eyelid from elevating or cause an asymmetric appearance. Some causes include blepharochalasis, chalazion, orbital fat prolapse, eyelid tumors, blood product due to trauma and cicatricial changes of the palpebral conjunctiva. Therefore, a careful external ocular examination of the adnexa, eyelids and conjunctiva is recommended.3
Ptosis secondary to botulinum toxin A injections. Ptosis can occur after a botulinum toxin A injection of the glabellar complex. The glabellar complex includes the frontalis, procerus, corrugator supercilii, depressor supercilii and orbicularis oculi muscles. Glabellar complex injections are used for cosmetic purposes and in the treatment of chronic migraines.7 Post-botulinum toxin A ptosis can occur anywhere between two days and 10 days later, with the ptosis lasting anywhere from two to four weeks after injection.8
Ptosis with Diplopia
Conditions such as autoimmune or mitochondrial diseases can result in ptosis with related double vision, two of which are described below.
Myasthenia gravis. This neuromuscular disease is an antibody-mediated autoimmune condition with improper communication in the neuromuscular junction (NMJ).9 Through a T-cell-dependent process, antibodies are created to attack the acetylcholine receptors in the NMJ resulting in decreased density of the receptors and abnormal morphology of the NMJ. MG affects voluntary muscles with ocular MG affecting the LPS, MM and EOMs. Pupillary muscle fibers are not affected, as they are not voluntary. However, EOMs are particularly susceptible to the effects of MG because the muscle fibers have a high frequency of synaptic firing when sustaining a fixated gaze. EOMs also have a lower density of acetylcholine receptors, resulting in faster onset weakness than in other skeletal muscles. In 50% of cases, ocular manifestations are the initial sign of MG.10
While diplopia and ptosis are the most common ocular manifestations of MG, they can occur independently of each other. When formulating history questions, keep in mind that the symptoms of diplopia and ptosis improve with rest. This results in variable ptosis or diplopia that can worsen by the end of the day.11 MG can be a systemic condition that becomes life-threatening if vital muscles are affected. The muscles of the esophagus may be affected, causing difficulty swallowing and increased risk of choking. If the diaphragm is affected, it can result in difficulty breathing and possible respiratory failure. Patients who present with difficulty breathing or swallowing should be sent to a hospital emergency department for treatment.3,11
EOMs affected by MG are variable and cover testing can mimic patterns consistent with cranial nerve palsies, gaze palsies and internuclear ophthalmoplegia; there may also be a nonspecific pattern. MG should be considered in patients with a presentation of both diplopia and ptosis. The cover test results and eyelid measurements may worsen with fatigue and improve with ice pack testing, since cooling increases the amount of acetylcholine in the NMJ by inhibiting acetylcholinesterase activity.12
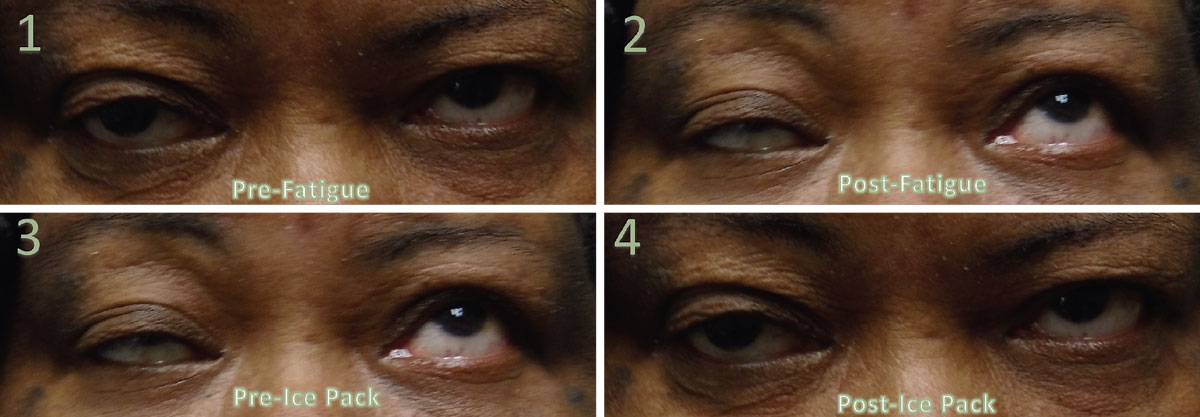
Before fatigue and ice pack testing, baseline ocular alignment, EOMs and eyelid measurements should be obtained. Fatigue and ice pack tests should be performed for two minutes each, with the fatigue test performed first. A 2mm or greater difference in pre- and post-palpebral aperture measurements is considered a positive result for both tests.11,13 Positive results are indicative of MG (Figure 2). Other tests suggestive of MG include orbicularis oculi weakness and a positive curtain test. These, however, are nonspecific for MG.11,13
 |
|
Fig. 2. These images represent a positive fatigue and ice pack test. Image 1 was taken prior to the fatigue testing. Image 2 was taken after two minutes of sustained up gaze. There was >2mm difference in the right palpebral aperture, indicating a positive test. Note the positive test occurred while up gaze was sustained. Image 3 depicts after fatigue testing, but prior to ice pack testing. Image 4 is after ice pack testing and is a positive result, as there is >2mm difference in the right palpebral aperture. Click image to enlarge. |
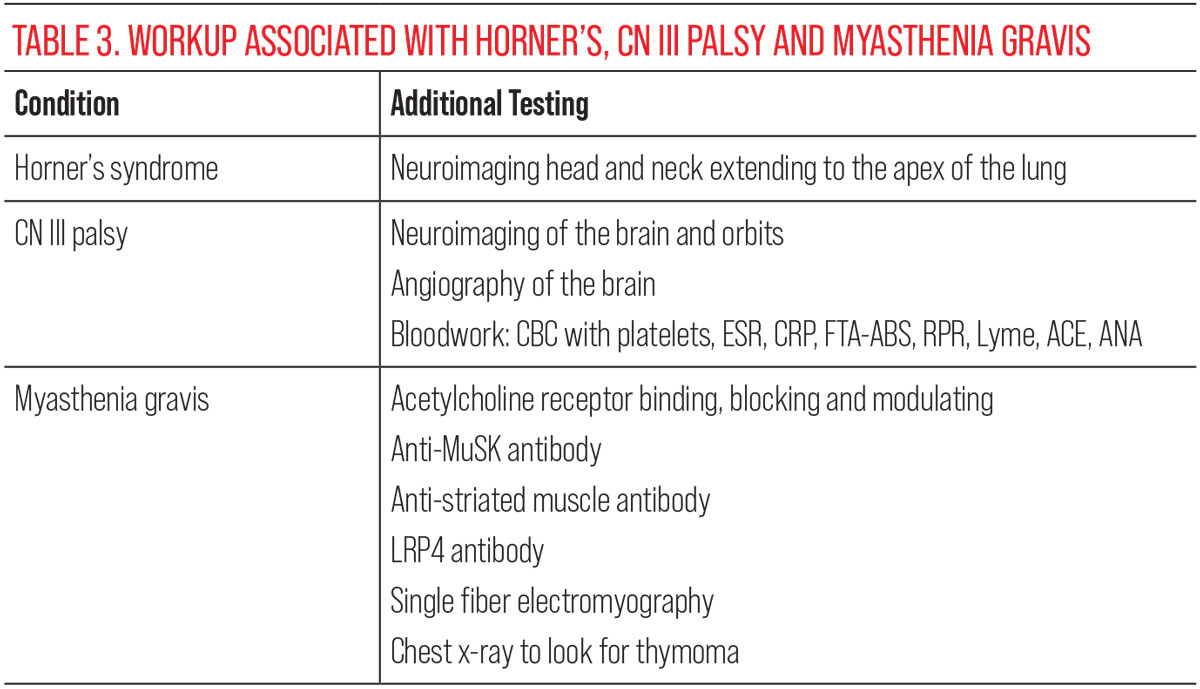
Blood tests are the most common way to test for MG. Acetylcholine receptor antibody titers have the highest positivity rate at 85% when suspecting MG, followed by anti-muscle specific kinase (MuSK) antibody, lipoprotein receptor-related antibody 4 (LRP4) and anti-striated muscle antibody. A stepwise approach is expected when ordering these blood tests, starting with acetylcholine receptor antibody (binding, blocking, modulating). If these tests are negative, anti-MuSK antibody, LRP4 antibody and anti-striated muscle antibody can be ordered if suspicion for MG is high; however, less than 10% of patients with MG will be seronegative for any of these antibodies.14 If this is the case, single fiber electromyography can be performed to confirm the MG diagnosis (Table 3).15
 |
| Click image to enlarge. |
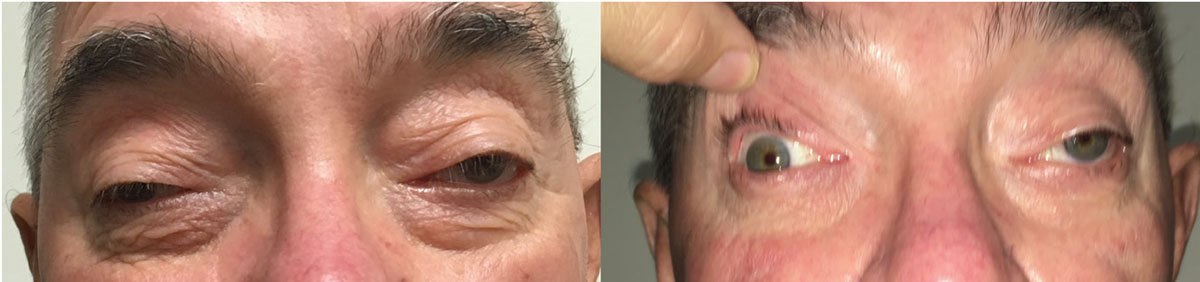
Chronic progressive external ophthalmoplegia. This genetic condition results in slowly developing ptosis and ophthalmoplegia. CPEO has been classified as a mitochondrial encephalomyopathy and can be confirmed with genetic testing. Since mitochondria are found in nearly every tissue, these patients may also present with other affected organ systems.16 Ocular presentation is typically asymmetric and becomes more symmetric as the disease progresses (Figure 3). CPEO affects all external ocular muscles, limiting all directions of gaze. Given the genetic correlation, eyecare providers should inquire about a family history of ptosis or ophthalmoplegia.17,18
Each case of CPEO is unique. Therefore, the cover test pattern can be nonspecific and may mimic other conditions. However, these patients will not have variability of ocular misalignment or ptosis, as is seen with MG. Patients with mitochondrial syndromes may present with other ocular and systemic findings as well; fundus examination may reveal optic disc atrophy or retinopathy with disruption of the retinal pigment epithelium. Comanagement with other specialties is necessary due to potential systemic involvement of mitochondrial syndromes.18
 |
|
Fig. 3. This photo represents a patient with chronic progressive external ophthalmoplegia. At left, he is looking in primary gaze without his lids held. At right, he is in primary gaze with lids held. Click image to enlarge. |
Ptosis with Miosis
Another class of ptosis, accompanied by pupil constriction, is mainly indicative of the neurological condition of Horner’s syndrome (HS).
This syndrome is caused by damage to the oculosympathetic pathway. Initiating in the hypothalamus, this pathway has a long ipsilateral course and consists of three neurons. Associated symptoms can assist in localization of the lesion within this pathway. The first neuron starts in the hypothalamus and travels down the dorsal portion of the brainstem and spinal cord to the level of T1, where it synapses in the ciliospinal center of Budge and exits the central nervous system.19,20 The second neuron ascends the cervical sympathetic chain, travels over the apex of the lung and synapses at the level of C3 to C4 in the superior cervical ganglion, between the internal carotid artery and internal jugular vein. The final neuron travels with the internal carotid artery into the skull base, through the cavernous sinus, then joins the ophthalmic division of the trigeminal nerve to enter the orbit through the superior orbital fissure. The oculosympathetic nerve ultimately innervates the MM and the iris dilator muscle.19-21
HS classically presents with ptosis, miosis and anhidrosis, but anhidrosis is not a common symptom reported by patients. MM assists in the elevation of the eyelid, but the primary muscle is the LPS. Therefore, ptosis associated with Horner’s is typically smaller than when involving the LPS, only about 2mm to 3mm. Reverse ptosis may also be present due to damage of the sympathetic innervation of the lower eyelid MM.19 Anisocoria is expected in HS patients and is typically greater in dim illumination. Pictures taken with an infrared camera can help observe anisocoria when measuring in dim conditions. Coexistent EOM abnormalities can help localize HS to the brainstem or cavernous sinus.20 For example, a patient with a cranial nerve IV palsy and an ipsilateral HS could localize to the cavernous sinus. In contrast, a patient with a cranial nerve IV palsy and a contralateral HS can localize to the midbrain. Inquiries about headaches and neck pain are vital because painful HS is a medical emergency because of concern for a carotid artery dissection.22
Horner’s syndrome should be considered in all patients with ipsilateral miosis and ptosis. To confirm HS, a diagnostic test can be performed on an untouched cornea; no ocular drops should be instilled before this testing. Cocaine 2% to 10% is highly effective at confirming HS and is considered positive when anisocoria becomes greater within 45 minutes. The unaffected pupil will dilate while the pupil with HS will fail to do so in response to cocaine.22 Because it is a controlled substance, though, cocaine is difficult to obtain.
 |
|
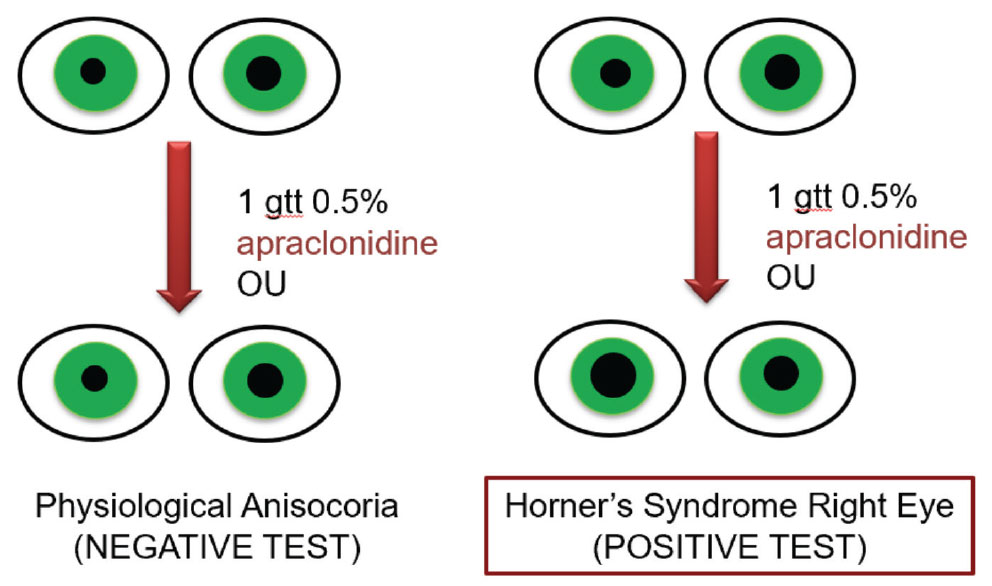
Fig. 4. A negative and positive response to 0.5% apraclonidine as related to the diagnosis of Horner’s syndrome. In a positive result, anisocoria are expected to reverse with 0.5% apraclonidine, while in a negative result, there will be no reversal of anisocoria. Click image to enlarge. |
Apraclonidine 0.5%, an alpha-2 adrenergic agonist, is more easily accessible and more commonly used to confirm Horner’s. A positive result can present up to an hour after drop instillation, occurring when the eye with the HS dilates in response, causing a reversal of anisocoria (Figure 4).23 Alpha-2 adrenergic agonists are contraindicated in children under six years old, as administration can result in bradycardia, hypotension, lethargy and somnolence. Therefore, cocaine is the preferred method for diagnostic testing in children with suspected HS.24
Hydroxyamphetamine and phenylephrine are agents that can be used to localize HS. However, these tests also need to be performed on an untouched cornea. Therefore, this testing must be completed at least 72 hours after initial confirmatory testing.25,26 After HS confirmation without known etiology, neuroimaging should be ordered. A magnetic resonance imaging (MRI) or computerized tomography scan (CT) of the head and neck with and without contrast should be performed. If possible, these images should extend to the apex of the lung to assess for any lesions along the oculosympathetic pathway (Table 3). When ordering neuroimaging for HS, consultation with radiology can help to ensure the most appropriate combination of studies are ordered.
Ptosis with Mydriasis and Diplopia
A ptosis presentation with both double vision and dilated pupils is often indicative of a cranial nerve III palsy.
The third cranial nerve originates in the medial portion of the midbrain in the oculomotor complex. As it exits the midbrain, caudal to the mammillary bodies, it passes between the posterior cerebral and superior cerebellar arteries, running adjacent to the posterior communicating artery before traveling through the lateral wall of the cavernous sinus. After exiting the cavernous sinus, CN III splits into the superior and inferior divisions before entering the orbit through the superior orbital fissure.27 The superior division of CN III innervates the superior rectus and LPS. By contrast, the inferior division innervates the inferior rectus, medial rectus and inferior oblique. The inferior division is also accompanied by the parasympathetic postganglionic fibers. These provide pupillary input to the iris sphincter and ciliary muscle.28
 |
|
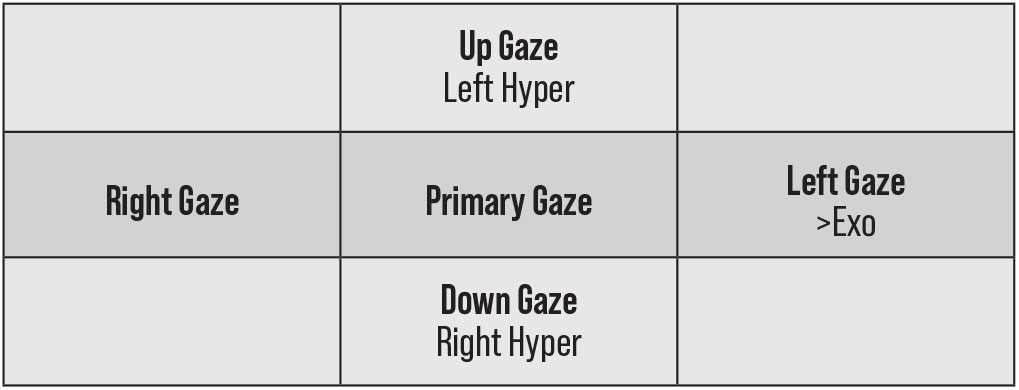
Fig. 5. This represents a pattern of right CN III palsy. There is a left hyper-deviation in up gaze with a right hyper-deviation in down gaze. This is representative of a reversing hyper-deviation. There is also an increasing exodeviation in left gaze. The opposite would be true of a left CN III palsy. Click image to enlarge. |
If the LPS is affected in either a partial or complete CN III palsy, it can result in any degree of ptosis. Since CN III innervates most EOMs, diplopia is a common complaint. However, patients who present with a more prominent ptosis from a CN III palsy may not complain of diplopia, as the eyelid may be covering the visual axis. It is commonly thought that the eye will be “down and out;” however, this may not be true for subtler palsies. The cover test pattern that should raise concern for a CN III palsy is a reversing hyper-deviation in up and down gazes with increased exodeviation in the gaze, contralateral to the affected eye (Figure 5).
A partial or complete CN III palsy could result from damage anywhere along the pathway of CN III; compressive, ischemic or vasculopathic etiologies are most common.29 A vasculopathic etiology is suspected with conditions of diabetes, hypertension and hyperlipidemia, but is a diagnosis of exclusion and should not be assumed without further investigation.
Neuroimaging is needed to assess for a compressive lesion when evaluating CN III palsies. This will include an MRI or CT scan of the brain and orbits both with and without contrast and magnetic resonance imaging of the arteries (MRA) or computerized tomography of the arteries (CTA). CN III palsies can also be due to infectious and inflammatory etiologies, with a complete blood count including platelets, C-reactive protein and erythrocyte sedimentation rate helping assess for giant cell arteritis, generalized infections and anemia. Specific infectious etiologies, such as syphilis and Lyme disease, should additionally be investigated. Autoimmune conditions and other infectious processes, such as sarcoidosis, should be ruled out (Table 3).30,31 If the patient has no known vasculopathic conditions, risk factors for such should be assessed with blood pressure monitoring and lab testing for diabetes and hyperlipidemia.20
Vasculopathic CN III palsy occurs due to compromised vasa nervorum blood supply to the internal aspect of the nerve, sparing the more superficial pupillary fibers. These etiologies tend to improve on their own in about three months.29 Not as forgiving, a compressive CN III palsy can cause ipsilateral mydriasis due to damage of the parasympathetic pupillary fibers running on the outer surface of the nerve. This can result in greater anisocoria in bright illumination. A pupil-involving CN III palsy is particularly concerning for a posterior communicating artery aneurysm.29,32 All patients with a new CN III palsy, especially those with pupillary involvement or pain, should be sent to the hospital emergency department to rule out aneurysm with MRA or CTA.33
Treatment
When treating ptosis, it is essential to first treat any underlying systemic condition. Diseases such as MG and CPEO should be comanaged with other specialties like neurology, cardiology, endocrinology and rheumatology, dependent upon the patient’s clinical presentation. In patients who complain of the cosmesis of ptosis, there are topical medications and surgical options. Topical ophthalmic medications include oxymetazoline hydrochloride 0.1% and off-label use of apraclonidine 0.5%.
These medications are alpha-adrenergic agonists and are thought to stimulate the sympathetic nervous system, resulting in a contraction of MM and elevation of the upper eyelid by approximately 2mm.34,35 Oxymetazoline hydrochloride is used once daily and takes five to fifteen minutes to take effect. This medication can be used on a daily basis, or as needed based on the patient’s desire to improve the cosmetic appearance of their ptosis.36 Oxymetazoline hydrochloride should be avoided in patients with cardiovascular disease and narrow angles. Side effects include ocular surface dryness and irritation after drop instillation.36 For patients with long-standing prominent ptosis, surgical intervention may be indicated, except in patients with variable or progressive ptosis. Referral to an oculoplastic specialist can be needed for surgical intervention.37
 |
|
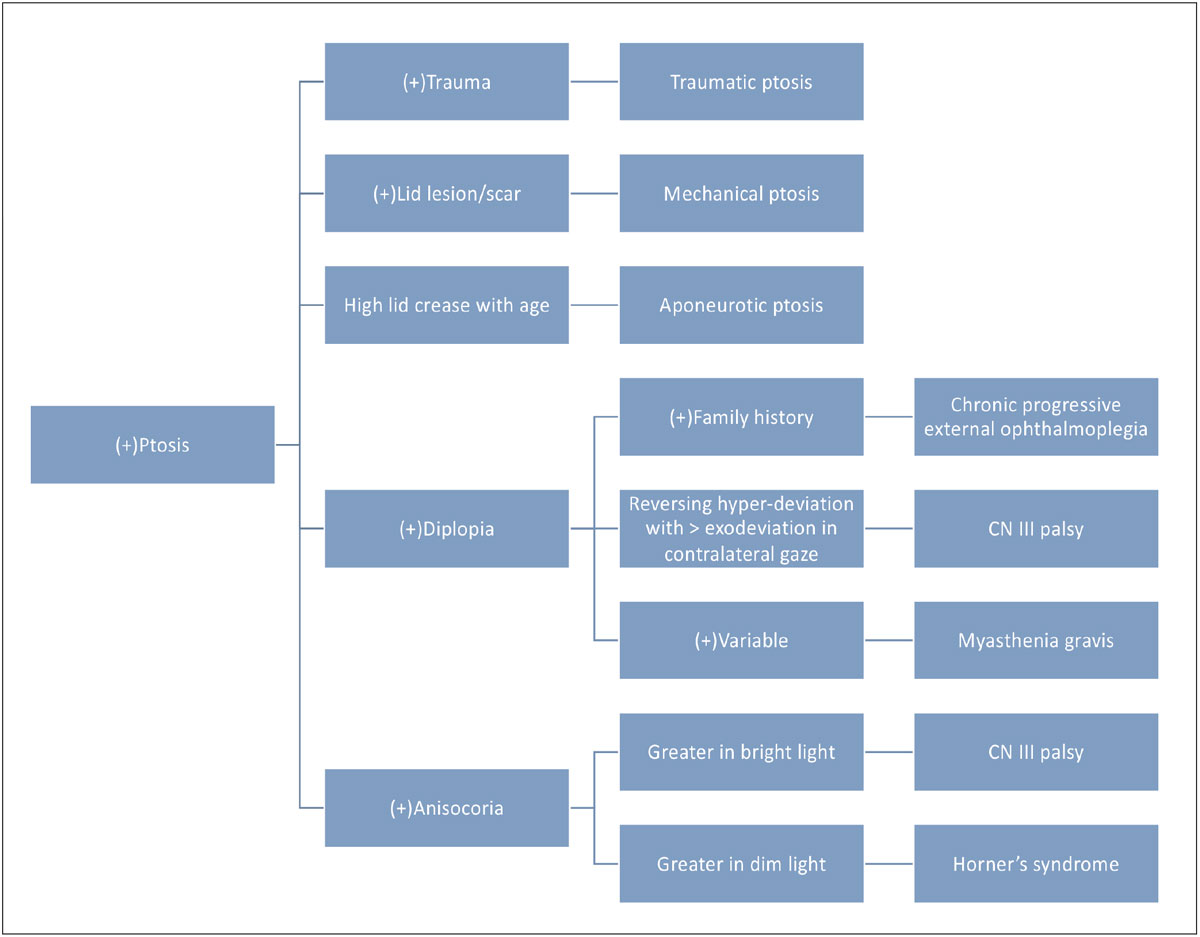
Fig. 6. The flowchart above represents the common differentials of ptosis (not an all-inclusive list). Click image to enlarge. |
Takeaways
It is common for initial ptosis evaluations to be performed by optometrists, although outside care may be necessary, depending on evaluation results. Not all patients will be aware of their ptosis; optometrists may be the first to take note of the finding, especially in subtler cases. While there are many etiologies of acquired ptosis, optometrists can incorporate case history, examination techniques and additional testing to differentiate and diagnose associated conditions. Comanagement with other specialties should be considered in conditions with potential systemic involvement.
Dr. Marunde completed her doctorate of optometry at University of the Incarnate Word Rosenberg School of Optometry in San Antonio, Texas. She completed a two-year residency in neuro-ophthalmic disease at Salus University, Pennsylvania College of Optometry, where she is currently an instructor.
1. Shahzad B, Siccardi MA. Ptosis. In: StatPearls. Treasure Island (FL): StatPearls Publishing. Updated February 19, 2023. 2. Alotaibi GF, Alsukait SF, Alsalman HH, Turkmani MG. Eyelid ptosis following botulinum toxin injection treated with brimonidine 0.33% topical gel. JAAD Case Rep. 2022;22:96-8. 3. Finsterer J. Ptosis: causes, presentation, and management. Aesthetic Plast Surg. 2003;27(3):193-204. 4. Koka K, Patel BC. Ptosis correction. In: StatPearls. Treasure Island (FL): StatPearls Publishing. Updated July 10, 2023. 5. Lim JM, Hou JH, Singa RM, Aakalu VK, Setabutr P. Relative incidence of blepharoptosis subtypes in an oculoplastics practice at a tertiary care center. Orbit. 2013;32(4):231-4. 6. Jacobs SM, Tyring AJ, Amadi AJ. Traumatic ptosis: evaluation of etiology, management, and prognosis. J Ophthalmic Vis Res. 2018;13(4):447-52. 7. FDA approved medication guide: BOTOX (onabotulinumtoxinA). FDA. Published 2011. accessdata.fda.gov/drugsatfda_docs/label/2011/103000s5232lbl.pdf. Accessed July 11, 2023. 8. Klein AW. Complications, adverse reactions and insights with the use of botulinum toxin. Dermatol Surg. 2003;29(5):549-56. 9. Dresser L, Wlodarski R, Rezania K, Soliven B. Myasthenia gravis: epidemiology, pathophysiology and clinical manifestations. J Clin Med. 2021;10(11):2235. 10. Grob D, Arsura EL, Brunner NG, Namba T. The course of myasthenia gravis and therapies affecting outcome. Ann N Y Acad Sci. 1987;505:472-99. 11. Nair AG, Patil-Chhablani P, Venkatramani DV, Gandhi RA. Ocular myasthenia gravis: a review. Indian J Ophthalmol. 2014;62(10):985-91. 12. Sethi KD, Rivner MH, Swift TR. Ice pack test for myasthenia gravis. Neurology. 1987;37(8):1383-5. 13. Keane JR. Vertical diplopia. Semin Neurol. 1986;6(2):147-54. 14. Lazaridis K, Tzartos SJ. Autoantibody specificities in myasthenia gravis; implications for improved diagnostics and therapeutics. Front Immunol. 2020;11:212. 15. Rivero A, Crovetto L, Lopez L, et al. Single fiber electromyography of extraocular muscles: a sensitive method for the diagnosis of ocular myasthenia gravis. Muscle Nerve. 1995;18(9):943-7. 16. Deschauer M, Müller T, Dreha S, Zierz S. [Familial mitochondrial chronic progressive external ophthalmoplegia. Five families with differing genetics]. Nervenarzt. 2001;72(2):122-9. 17. Caballero PEJ, Candela MS, Alvarez CIC, Tejerina AA. Chronic progressive external ophthalmoplegia: a report of six cases and a review of the literature. Neurologist. 2007;13(1):33-6. 18. Bau V, Zierz S. Update on chronic progressive external ophthalmoplegia. Strabismus. 2005;13(3):133-42. 19. Walton KA, Buono LM. Horner syndrome. Curr Opin Ophthalmol. 2003;14(6):357-63. 20. Sanders M. Walsh and Hoyt’s clinical neuro-ophthalmology. 4th ed., volume 5. J Neurol Neurosurg Psychiatry. 1996;61(2):236-7. 21. Reede DL, Garcon E, Smoker WRK, Kardon R. Horner’s syndrome: clinical and radiographic evaluation. Neuroimaging Clin N Am. 2008;18(2):369-85. 22. Kardon RH, Denison CE, Brown CK, Thompson HS. Critical evaluation of the cocaine test in the diagnosis of Horner’s syndrome. Arch Ophthalmol. 1990;108(3):384-7. 23. Bremner F. Apraclonidine is better than cocaine for detection of Horner syndrome. Front Neurol. 2019;10:55. 24. Al-Shahwan S, Al-Torbak AA, Turkmani S, et al. Side-effect profile of brimonidine tartrate in children. Ophthalmology. 2005;112(12):2143. 25. Danesh-Meyer HV, Savino P, Sergott R. The correlation of phenylephrine 1% with hydroxyamphetamine 1% in Horner’s syndrome. Br J Ophthalmol. 2004;88(4):592-3. 26. Wilhelm H, Welhelm B, Kriegbaum C. Interaction of the indirectly acting topical sympathomimetics cocaine and pholegrine. Ger J Ophthalmol. 1996;5:168-70. 27. Flanders M, Hasan J, Al-Mujaini A. Partial third cranial nerve palsy: clinical characteristics and surgical management. Can J Ophthalmol. 2012;47(3):321-5. 28. Joyce C, Le PH, Peterson DC. Neuroanatomy, cranial nerve 3 (oculomotor). In: StatPearls. Treasure Island (FL): StatPearls Publishing. Updated March 27, 2023. 29. Keane JR. Third nerve palsy: analysis of 1400 personally-examined inpatients. Can J Neurol Sci. 2010;37(5):662-70. 30. Appenzeller S, Veilleux M, Clarke A. Third cranial nerve palsy or pseudo third nerve palsy of myasthenia gravis? A challenging diagnosis in systemic lupus erythematosus. Lupus. 2009;18(9):836-40. 31. Kaiser PK, Friedman NJ, Pineda R. Third cranial nerve palsy. The Massachusetts eye and ear infirmary illustrated manual of ophthalmology. 4th ed. Saunders Elsevier, 2014:83-87. 32. Capó H, Warren F, Kupersmith MJ. Evolution of oculomotor nerve palsies. J Clin Neuroophthalmol. 1992;12(1):21-5. 33. Motoyama Y, Nonaka J, Hironaka Y, Park YS, Nakase H. Pupil-sparing oculomotor nerve palsy caused by upward compression of a large posterior communicating artery aneurysm. Case report. Neurol Med Chir (Tokyo). 2012;52(4):202-5. 34. Morales J, Brown SM, Abdul-Rahim AS, Crosson CE. Ocular effects of apraclonidine in Horner syndrome. Arch Ophthalmol. 2000;118(7):951-4. 35. Bacharach J, Wirta DL, Smyth-Medina R, et al. Rapid and sustained eyelid elevation in acquired blepharoptosis with oxymetazoline 0.1%: randomized Phase 3 trial results. Clin Ophthalmol. 2021;15:2743-51. 36. Upneeq [package insert]. Bridgewater, NJ: RVL Pharmaceuticals, INC.; 2020. 37. McCord CD, Tanenbaum M, Nunery WR. Oculoplastic surgery. New York: Lippincott, Williams and Wilkins, 1995. 38. Patil SB, Kale SM, Math M, Khare N, Sumeet J. Anthropometry of the eyelid and palpebral fissure in an Indian population. Aesthet Surg J. 2011;31(3):290-4. 39. Rana K, Beecher MB, Caltabiano C, et al. Normal periocular anthropometric measurements in an Australian population. Int Ophthalmol. 2023;43:2695-2701. 40. Migliori ME, Gladstone GJ. Determination of the normal range of exophthalmometric values for black and white adults. Am J Ophthalmol. 1984;98(4):438-42. |

