Age-related macular degeneration (AMD) is the leading cause of vision loss in patients over the age of 65.1 As there is no cure for AMD, current management focuses on reducing risk of conversion to advanced stages with formation of geographic atrophy (GA; advanced non-exudative AMD) or choroidal neovascularization (CNV; advanced exudative AMD). In addition, early detection of CNV is crucial in obtaining best visual outcomes for those who develop exudative AMD.
Development of advanced-stage disease poses the greatest threat of acuity reduction and significant loss of central vision, but even those without advanced disease may suffer visual deficits such as reduction in dark adaptation and loss of contrast sensitivity.2-6
Optometrists can manage patients with dry AMD from a medical perspective while also providing optical and low vision services to meet their visual needs. This article will focus on the medical management of those with non-exudative AMD by answering five common questions.
How Can I Tackle Compliance?
This perennial problem in many spheres of care is certainly a factor in AMD as well.
Knowledge is key. Patients who understand their condition and reason for being monitored are more apt to adhere to a mutual agreement. Those who are completely asymptomatic may not understand why they are monitoring their vision at home or why they are having periodic follow-ups when they have no visual symptoms.
On the other hand, those with reduced visual function may not understand why they are required to have office visits periodically when nothing is being done to “cure” their disease. They must understand the limitations that exist when managing AMD and the reason why we are monitoring them and making certain recommendations in order for them to adhere to follow-up.
A conversation about AMD and CNV may start off like this:
“AMD is a degenerative condition of the retina where the retina ages more quickly than it should. This accelerated aging can lead to loss of visual function as the retina structures weaken and no longer function normally.”
“There is no cure for AMD, but I am going to make specific recommendations that will decrease your risk of vision loss. Many patients with AMD maintain functional vision for a lifetime, and while AMD can affect your central vision, it does not make you go completely blind because peripheral vision is still intact.”
 |
|
Fig. 1. Teach your patient the retina basics. Show them imaging of their retina to help them understand their disease. Click image to enlarge. |
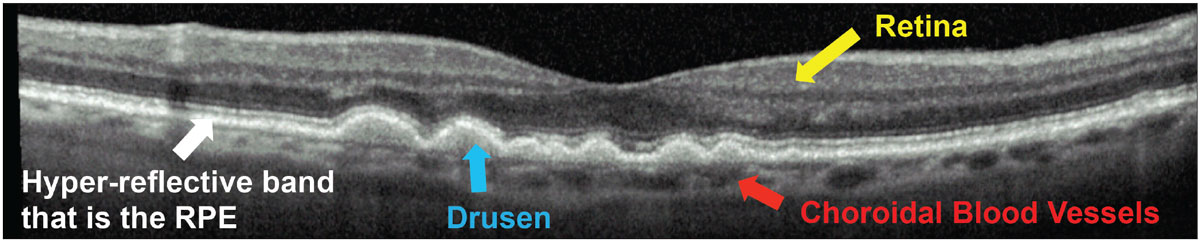
Visualize the problem. It is very useful for patients to be able to visualize alterations to their macula. Show them their fundus photographs or optical coherence tomography (OCT) images and walk them through the abnormalities that exist. Consider comparing them with normal photos or OCT scans.
I show patients with drusen their OCT images and point out that AMD affects the bottom layer of the retina (showing them the hyper-reflective band that is the retinal pigmented epithelium [RPE]), causing buildup of waste products (showing them areas of drusen). I explain that this layer of the retina is a barrier between the retina above and blood vessels (the choroid) underneath. This layer is weakened in patients with AMD, and these blood vessels can grow through and start to leak or bleed, causing a sudden and severe decline in vision (Figure 1). Although this is very simplified pathophysiology, it can help patients understand why they must be monitored daily for conversion to wet AMD, even though they may be asymptomatic.
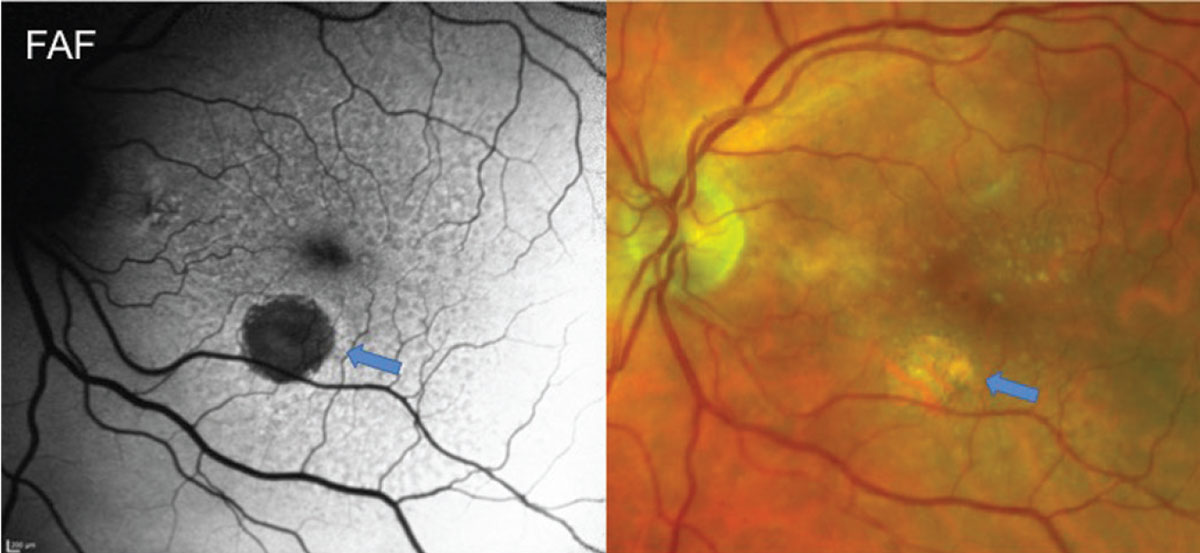
In patients who have GA, fundus autofluorescence (FAF) can demonstrate how certain areas of the retina have atrophied, causing the vision to have “good spots” and “bad spots” (Figure 2). Patients can understand visual deficits better and see that, while they may see 20/20 on the acuity chart, there are areas of the vision in with scotomas. On FAF, regions of GA will appear dark or hypo-autofluorescent.7
 |
|
Fig. 2. Fundus autofluorescence and fundus photo of a patient with reticular pseudodrusen and GA. FAF highlights a hypo-autofluorescent region of GA (blue arrows) and shows diffuse RPE alterations from RPD. FAF in patients with RPD often shows more diffuse disease than can be seen clinically with a reticular pattern of circular areas of hypo-autofluorescence often with a hyper-autofluorescent center. Click image to enlarge. |
In addition to traditional diagnostic imaging, the AdaptDx Pro is an FDA-approved instrument from Maculogix that can demonstrate altered function of the retina.8,9 This may help both the patient and the physician understand how the retina is functioning in those with good visual acuity but significant visual complaints. It can also convince those who mistakenly feel that their vision is fine. Dark adaptation may also be altered prior to any clinical findings.8
Keep an eye on them. Until recently, we had no way of monitoring our patients outside of their scheduled follow-ups. We could send them home with an Amsler grid but couldn’t determine whether they were using it or if they understood how to use it. Now there is an FDA-approved at-home monitoring system for those who have intermediate AMD, the ForeseeHome system from Notal Vision. It takes the guesswork out of vision monitoring. Patients test their vision at home daily with a physician-prescribed monitoring device. Algorithms use hyperacuity to detect visual changes consistent with conversion to exudative AMD.
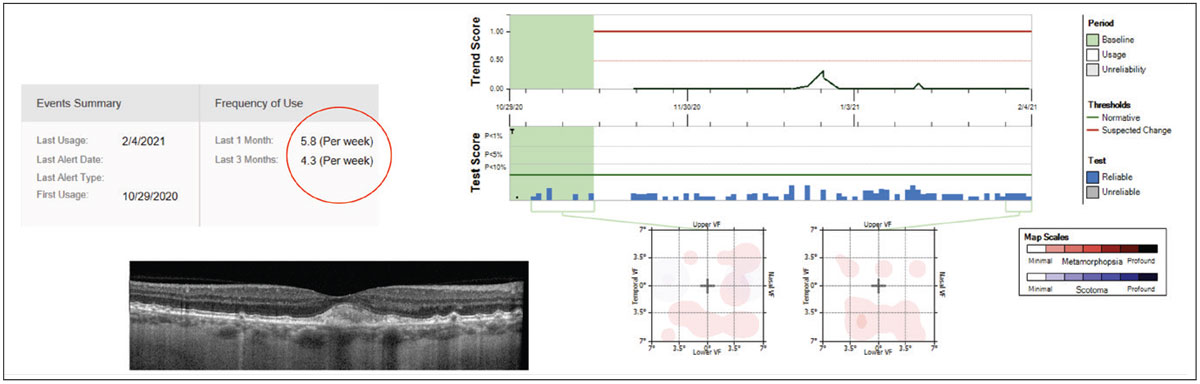
One report showed that 94% of eyes using ForeseeHome maintained 20/40 vision or better after conversion to exudative AMD vs. only 62% using current standard-of-care methods such as the Amsler grid.10 This is due to earlier detection with ForeseeHome. If conversion is detected, the prescribing physician will be alerted to contact the patient immediately. Physicians can access testing history through a portal or an app on their phone to know how often patients are testing, keeping them accountable (Figure 3).
When Should I Employ an Amsler Grid or Other At-home Monitoring Options?
Conversion to wet AMD remains the greatest risk for severe vision loss in those with AMD. Even with anti-VEGF injections, earlier detection of CNV leads to better visual outcomes. We rely heavily on home monitoring techniques and self-reporting to catch these conversions that can happen between visits. Educate patients with any stage of AMD about at-home vision monitoring with tools such as the Amsler grid. Those with intermediate AMD also qualify for the at-home vision monitoring system, ForeseeHome. Heavily consider that option for those with intermediate AMD.
 |
|
Fig. 3. On this ForeseeHome system report, a patient was testing regularly with almost six tests performed per week in the last month (red circle). I felt more comfortable then to extend his follow-up to six months. Click image to enlarge. |
Patients may ask, “Do I really need this device at my stage?” This is a major checkpoint to determine whether patients truly understand why they are being monitored. Introduce the program as AMD insurance. You hope to never use it, but it is there if you need it.
Figure 4 shows conversion to wet AMD that occurred less than three weeks after a scheduled monitoring appointment in a patient being followed for advanced non-exudative AMD. This well-educated patient noticed new-onset visual distortion of her Amsler grid and called our office. She was immediately brought in that afternoon where OCT confirmed presence of exudation, and treatment with anti-VEGF was initiated.
 |
|
Fig. 4. A patient with advanced dry AMD was seen with 20/20 OS. Less than three weeks later, the patient self reported visual changes and was brought into the clinic. OCT confirmed conversion to wet AMD OS and 20/25 vision (bottom). Click image to enlarge. |
Anecdotal stories and images may be useful to share with other AMD patients. By sharing your personal experiences in managing AMD, it makes the disease seem much more relatable and less abstract for the newly diagnosed patient. It also emphasizes the importance of at-home monitoring.
When Should I Decide to Discuss Lifestyle Interventions?
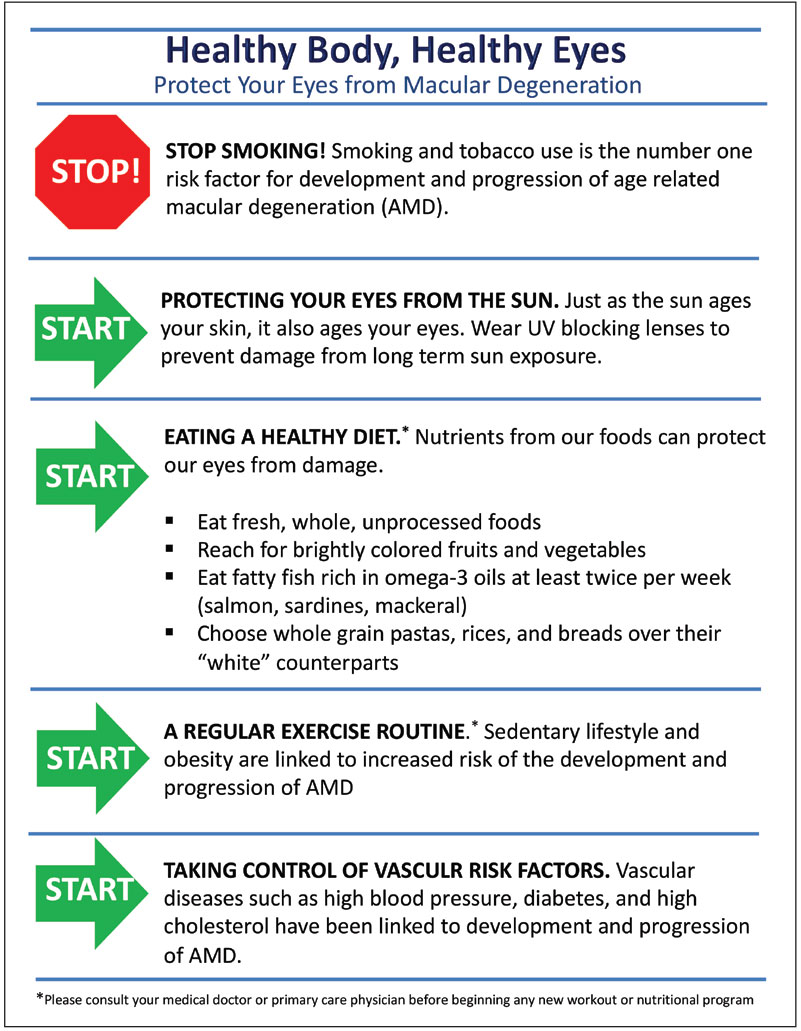
Simply put, the sooner the better. Recommending healthy diet, exercise, smoking cessation and UV protection is generally a positive thing for any patient, but especially those with risk factors such as family history of AMD, systemic vascular disease, poor nutrition and history of smoking. Discuss with them how such factors increase risk of macular degeneration and how lifestyle modifications can decrease it.
 |
|
Fig. 5. Give your patients a reference sheet of AMD lifestyle modifications such as this one. Click image to enlarge. |
Smoking remains the most important modifiable risk factor for development and progression of AMD.11,12 Many patients know that smoking can be bad for their heart or for their lungs, but it often comes as a surprise that it can also increase their risk of losing vision.
Making dietary recommendations can be daunting for those who are not used to this type of conversation. Even simple recommendations can spark a change for patients. One easy recommendation for patients can be to eat more naturally sourced food. Real, whole, unprocessed foods with a focus on brightly colored fruits and vegetables is a good place to start.
Additional suggestions may include observing a Mediterranean-type diet that involves intake of fatty fish rich in omega-3 oils at least twice per week and incorporating whole grain forms of rice, pasta and bread.13 For those with complicated systemic health, consider comanagement with the primary care physician or a nutritionist to facilitate dietary improvements.
Educate patients on the correlation between AMD and a sedentary lifestyle, obesity and vascular diseases such as hypertension, diabetes and hyperlipidemia.14 Consider making or using a quick reference sheet of the AMD lifestyle do’s and don’ts to give to your patients as shown in Figure 5.
When Should I Recommend Vitamin Supplementation?
Nutritional supplementation remains controversial. So, unfortunately, there is not an easy or straightforward answer. For a disease with no cure and very limited treatment options, there is a desire to do everything possible for a patient. This has to be appropriately balanced with current evidence. Recommendations for vitamin supplementation must always consider potential adverse effects alongside potential benefits.
The following is quoted from the American Academy of Ophthalmology’s Preferred Practice Patterns: “Antioxidant vitamin and mineral supplementation as per the Age-Related Eye Disease Study (AREDS II) should be considered in patients with intermediate or advanced AMD. There is no evidence to support the use of these supplements for patients who have less than intermediate AMD and no evidence of any prophylactic value for family members without signs of AMD.”15
 |
|
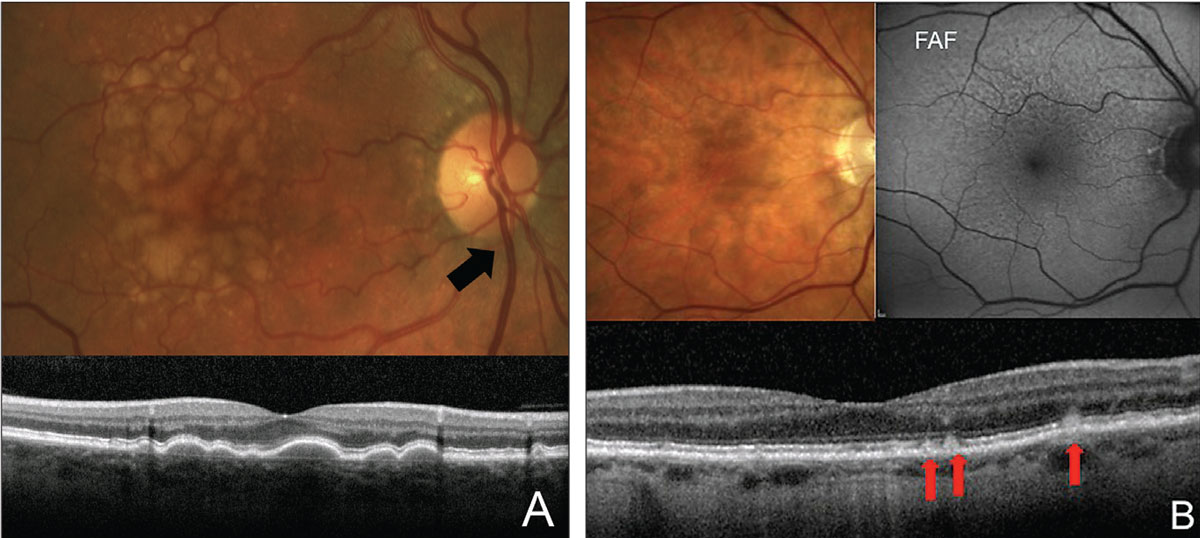
Fig. 6. Look out for the following clinical findings: large soft drusen (A) and RPD (B). Soft drusen are the width of a major retinal vein as it crosses the disc (black arrow). RPDs are more evident on FAF and OCT. On OCT, they present as hyper-reflective deposits on top of the RPE (red arrows). Click image to enlarge. |
The authors of the AREDS I trial concluded that clinicians should recommend AREDS-type supplementation only for those with intermediate AMD.16 Because there has yet to be a separate conclusive body of evidence that any type of supplementation reduces the risk of AMD progression or development in those with early AMD or those who do not yet have AMD, most guidelines still adhere to this recommendation.
The AREDS II trial concluded that use of supplements containing the following should be recommended for patients with intermediate AMD: 10mg lutein/2mg zeaxanthine, 500mg vitamin C, 400 IU vitamin E, 80mg zinc and 2mg copper.17 While AREDS II did not find benefit of docosahexaenoic acid and eicosapentaenoic acid, there is other evidence that shows potential benefits of omega-3 fatty acids.18-20 Many over-the-counter supplements do contain these ingredients.
Discussing whether to recommend a particular vitamin supplement of the optometrist’s choosing to patients who have intermediate AMD may go like this:
“There is evidence, for your stage of macular degeneration, that vitamin supplements do help to reduce the risk of developing more advanced stages of macular degeneration by 25%. I am going to recommend a supplement for you. This does not take the place of a healthy diet and lifestyle that involves wearing sunglasses and discontinuing smoking, which are equally important!”
What about those with early AMD or at-risk patients? While there is no substantial proof that vitamin supplementation reduces the risk of AMD progression or development in those with early-stage disease or at-risk patients such as those whose family members have AMD, there are multiple trials that suggest benefit to macular pigment optical density as well as both objective and subjective measures of visual function including visual acuity, contrast sensitivity and electroretinography with various types of vitamin supplementation.21-29
Supplementation with carotenoids such as lutein, zeaxanthin and meso-zeaxanthin seems reasonable in this patient population, particularly in those with poor nutrition or visual limitations such as difficulty seeing at night.
The discussion with this patient population about vitamin supplementation may go as follows:
“While there is limited evidence that vitamin supplementation slows down the development/progression of macular degeneration at your stage, there is evidence to show that these vitamins may help the retina function more normally. I am going to recommend a supplement for you.”
How Should I Modify the Follow-up Schedule in Response to Presentation?
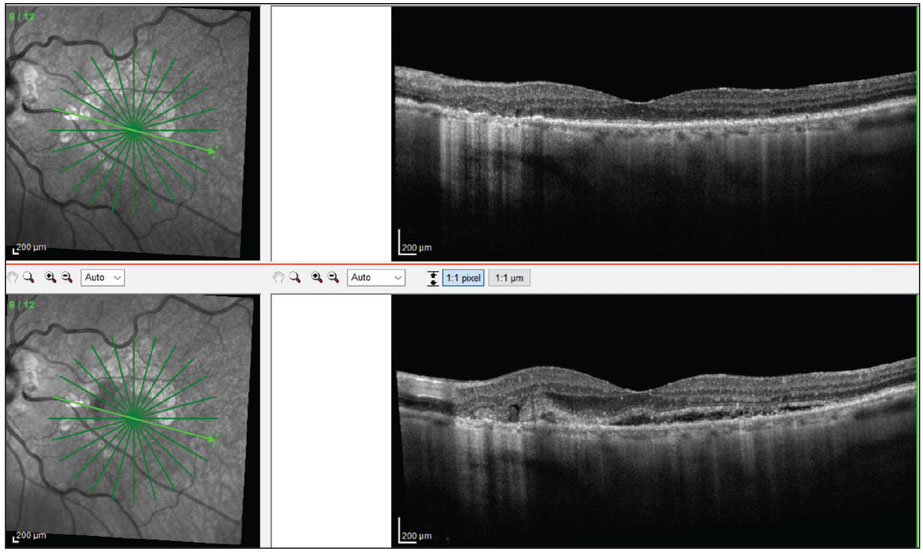
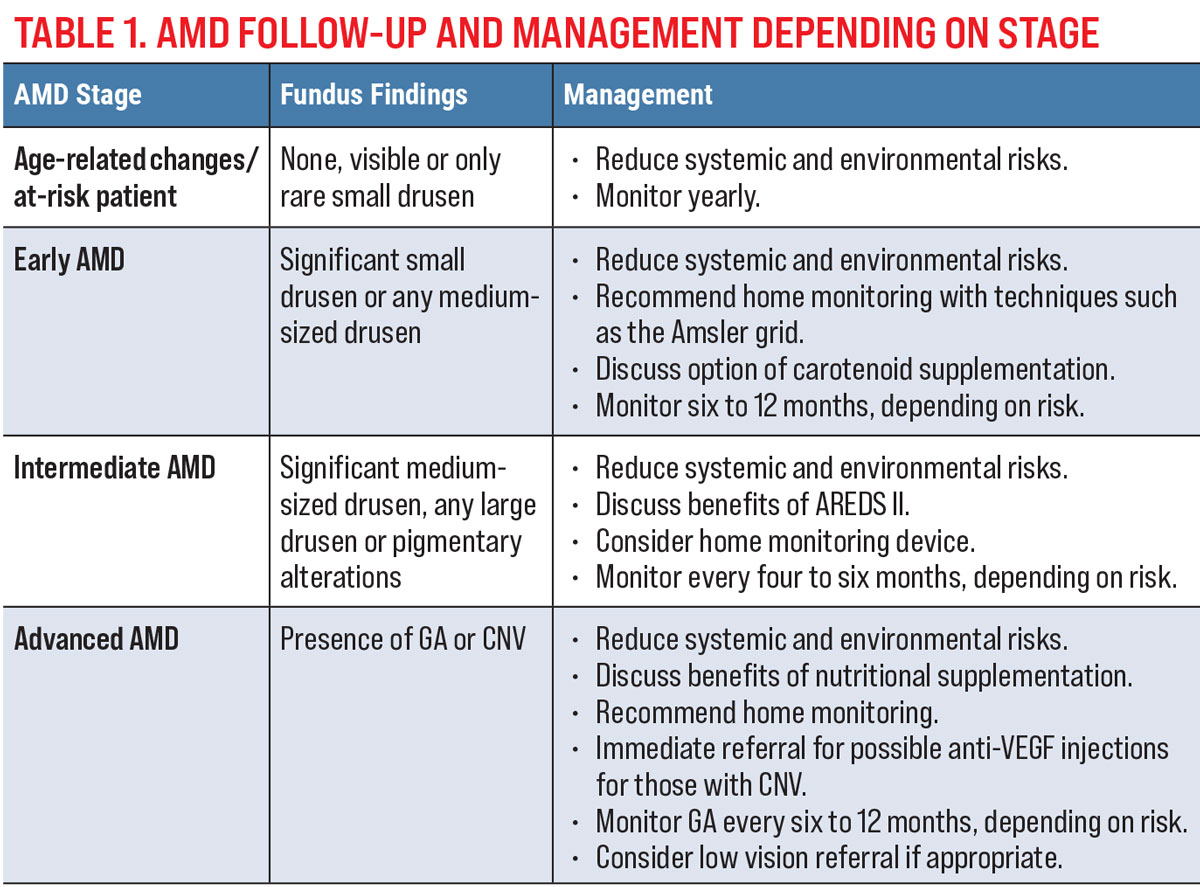
To plan follow-ups for your AMD patients, determine which clinical, OCT and FAF findings suggest increased risk for conversion to advanced AMD. A suggested follow-up and management depending on the stage of disease is shown in Table 1.
 |
| Click image to enlarge. |
It has been well documented that patients with any large-sized drusen 125nm or larger or with pigmentary changes on clinical exam are at increased risk for conversion to advanced AMD. This is approximately the width of a major retinal vein as it crosses the disc margin (Figure 6a). Those with both of these findings in each eye have a 50% chance of converting to advanced AMD in the next five years.30 The presence of either of these findings automatically puts a patient in the intermediate AMD category. It is highly important to make this distinction for three reasons.31 First, this is the point at which vitamin supplementation with full AREDS II-type vitamins is well accepted. Second, this is also when to use an at-home monitoring system. Third, it is pertinent to educate these patients and monitor them more carefully due to their increased risk of developing advanced AMD.
OCT imaging can be useful in visualizing small- to large-sized drusen and getting a sense of the size of the drusen deposits. In addition, it can help to detect a particular high-risk phenotypical variant of drusen deposition called reticular pseudodrusen (RPD), also known as subretinal drusenoid deposits. This variation is difficult to distinguish clinically and presents as small- to intermediate-sized drusen. On OCT, they appear as hyper-reflective deposits sitting on top of the RPE. Although small in size, patients with these deposits are even more likely than patients with large-sized drusen to develop advanced AMD.32-38
Also, these patients tend to be more symptomatic with worse visual function than other AMD phenotypes.39-41 Patients with RPD should be more heavily educated regarding increased risk of progression, need for nutritional and lifestyle modifications and need for more careful monitoring. FAF can also help to identify RPD (Figure 6b).
Key Clinical Takeaways
Optometrists often have the training, the knowledge and the access to all the tools necessary to manage patients with non-exudative AMD. Proper patient education, appropriate lifestyle, nutritional and nutraceutical recommendations, identification of risk factors and emphasis of both in-office and at-home monitoring can all lead to better visual outcomes for our patients with AMD.
Dr. Haynes is a consultative optometrist at the Charles Retina Institute and consulting faculty at Southern College of Optometry in Memphis, TN. She is a paid speaker for Heidelberg Engineering and a consultant for Notal Vision.
1. Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137(3):486-495. 2. Pondorfer SG, Wintergerst MWM, Gorgi Zadeh S, et al. Association of visual function measures with drusen volume in early stages of age-related macular degeneration. Investig Opthalmology Vis Sci. 2020;61(3):55. 3. Enger C, Alexander MF, Fine SL. Contrast sensitivity in age-related macular degeneration. Arch Ophthalmol. 1988;106(1):55-7. 4. Faria BM, Duman F, Zheng CX, et al. Evaluating contrast sensitivity in age-related macular degeneration using a novel computer-based test, the Spaeth/Richman contrast sensitivity test. Retina. 2015;35(7):1465-73. 5. Loshin DS, White J. Contrast sensitivity: the visual rehabilitation of the patient with macular degeneration. Arch Ophthalmol. 1984;102(9):1303-6. 6. Chung STL. Reading in the presence of macular disease: a mini‐review. Ophthalmic Physiol Opt. 2020;40(2):171-86. 7. Ly A, Nivison-Smith L, Assaad N, Kalloniatis M. Fundus autofluorescence in age-related macular degeneration. Optom Vis Sci. 2017;94(2):246-59. 8. Owsley C, McGwin G, Clark ME, et al. Delayed rod-mediated dark adaptation is a functional biomarker for incident early age-related macular degeneration. Ophthalmology. 2016;123(2):344-51. 9. Jackson GR, Scott IU, Kim IK, et al. Diagnostic sensitivity and specificity of dark adaptometry for detection of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55(3):1427-31. 10. Chew EY, Clemons TE, Bressler SB, et al; AREDS2-HOME Study Research Group. Randomized trial of a home monitoring system for early detection of choroidal neovascularization home monitoring of the Eye (HOME) study. Ophthalmology. 2014;121(2):535-44. 11. Makrynioti D, Zagoriti Z, Koutsojannis C, et al. Ocular conditions and dry eye due to traditional and new forms of smoking: a review. Cont Lens Anterior Eye. 2020;43(3):277-84. 12. Velilla S, García-Medina JJ, García-Layana A, et al. Smoking and age-related macular degeneration: review and update. J Ophthalmol. 2013;2013:895147. 13. Carneiro Â, Andrade JP. Nutritional and lifestyle interventions for age-related macular degeneration: a review. Oxid Med Cell Longev. 2017;2017:6469138. 14. Al-Zamil WM, Yassin SA. Recent developments in age-related macular degeneration: a review. Clin Interv Aging. 2017;12:1313-30. 15. AAO. Preferred Practice Pattern Guidelines. Age-Related Macular Degeneration. American Academy of Ophthalmology. 2019. 16. Kassoff A, Kassoff J, Buehler J, et al. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417-36. 17. Chew EY, Clemons TE, SanGiovanni JP, et al. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309(19):2005-15. 18. SanGiovanni JP, Agrón E, Meleth AD, et al. ω-3 long-chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: AREDS report 30, a prospective cohort study from the Age-Related Eye Disease Study. Am J Clin Nutr. 2009;90(6):1601-7. 19. Mance TC, Kovacević D, Alpeza-Dunato Z, et al. The role of omega-6 to omega-3 ratio in development and progression of age-related macular degeneration. Coll Antropol. 2011;35(2):307-10. 20. Lawrenson JG, Evans JR. Omega 3 fatty acids for preventing or slowing the progression of age-related macular degeneration. Cochrane Database Syst Rev. 2015;2015(4):CD010015. 21. Ma L, Dou HL, Huang YM, et al. Improvement of retinal function in early age-related macular degeneration after Lutein and zeaxanthin supplementation: a randomized, double-masked, placebo-controlled trial. Am J Ophthalmol. 2012;154(4):625-34.e1. 22. Beatty S, Chakravarthy U, Nolan JM, et al. Secondary outcomes in a clinical trial of carotenoids with coantioxidants versus placebo in early age-related macular degeneration. Ophthalmology. 2013;120(3):600-6. 23. Richer S, Devenport J, Lang JC. LAST II: differential temporal responses of macular pigment optical density in patients with atrophic age-related macular degeneration to dietary supplementation with xanthophylls. Optometry. 2007;78(5):213-9. 24. Richer S, Stiles W, Statkute L, et al. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry. 2004;75(4):216-29. 25. Evans JR, Lawrenson JG. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst Rev. 2017;2017(7):CD000254. 26. Parravano M, Tedeschi M, Manca D, et al. Effects of Macuprev supplementation in age-related macular degeneration: a double-blind randomized morpho-functional study along six months of follow-up. Adv Ther. 2019;36(9):2493-2505. 27. Falsini B, Piccardi M, Iarossi G, et al. Influence of short-term antioxidant supplementation on macular function in age-related maculopathy: a pilot study including electrophysiologic assessment. Ophthalmology. 2003;110(1):51-60. 28. Corvi F, Souied EH, Falfoul Y, et al. Pilot evaluation of short-term changes in macular pigment and retinal sensitivity in different phenotypes of early age-related macular degeneration after carotenoid supplementation. Br J Ophthalmol. 2017;101(6):770-3. 29. Bartlett H, Eperjesi F. Age-related macular degeneration and nutritional supplementation: a review of randomised controlled trials. Ophthalmic Physiol Opt. 2003;23(5):383-99. 30. Ferris FL, Davis MD, Clemons TE, et al. A simplified severity scale for age-related macular degeneration: AREDS Report no. 18. Arch Ophthalmol. 2005;123(11):1570-4. 31. Ferris FL, Wilkinson CP, Bird A, et al. Clinical classification of age related macular degeneration. Ophthalmology. 2013;120(4):844-51. 32. Joachim N, Mitchell P, Rochtchina E, et al. Incidence and progression of reticular drusen in age-related macular degeneration: findings from an older Australian cohort. Ophthalmology. 2014;121(4):917-25. 33. Gil JQ, Marques JP, Hogg R, et al. Clinical features and long-term progression of reticular pseudodrusen in age-related macular degeneration: findings from a multicenter cohort. Eye (Lond). 2017;31(3):364-71. 34. Pumariega NM, Smith RT, Sohrab MA, et al. A prospective study of reticular macular disease. Ophthalmology. 2011;118(8):1619-25. 35. Marsiglia M, Boddu S, Bearelly S, et al. Association between geographic atrophy progression and reticular pseudodrusen in eyes with dry age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54(12):7362-9. 36. Xu L, Blonska AM, Pumariega NM, et al. Reticular macular disease is associated with multilobular geographic atrophy in age-related macular degeneration. Retina. 2013;33(9):1850-62. 37. Finger RP, Wu Z, Luu CD, et al. Reticular pseudodrusen: a risk factor for geographic atrophy in fellow eyes of individuals with unilateral choroidal neovascularization. Ophthalmology. 2014;121(6):1252-6. 38. Sawa M, Ueno C, Gomi F, Nishida K. Incidence and characteristics of neovascularization in fellow eyes of Japanese patients with unilateral retinal angiomatous proliferation. Retina. 2014;34(4):761-7. 39. Ooto S, Ellabban AA, Ueda-Arakawa N, et al. Reduction of retinal sensitivity in eyes with reticular pseudodrusen. Am J Ophthalmol. 2013;156(6):1184-91.e2. 40. Ooto S, Suzuki M, Vongkulsiri S, et al. Multimodal visual function testing in eyes with non-exudative age-related macular degeneration. Retina. 2015;35(9):1726-34. 41. Querques G, Massamba N, Srour M, et al. Impact of reticular pseudodrusen on macular function. Retina. 2014;34(2):321-9. |

