 |
Whether you’re relatively new to treating glaucoma or you’re a well-seasoned clinician in this area of ophthalmic care, we’ve all seen a significant change in the technology used to evaluate and manage this ocular disease. Without a doubt, OCT has revolutionized glaucoma care, affording us the opportunity to visualize details of several structures—the optic nerve, retinal nerve fiber layer and ganglion cell layer—and detect change at the micron level.
Just as these significant advancements in technology have increased our ability to manage glaucoma, so has the expansion of optometric glaucoma care to include ODs in all 50 states. Adding to this is the aging population and the increasing incidence of glaucoma we expect to see throughout our careers.
With increased optometric scope of glaucoma care comes the responsibility of proper management, especially in more complex cases when the patient has advanced disease. In early to moderate glaucoma, since the disease progresses slowly in the majority of cases, there’s time to evaluate progression before initiating treatment. That safety window diminishes significantly when the patient has more advanced disease and an increased risk of further vision loss.
It is estimated that about 80% of glaucoma patients fall in the mild to moderate category, 10% in the advanced category and another 10% in the refractory category. With clinical experience and comprehensive education, ODs should be able to care for that 10% with advanced glaucoma, in addition to the 80% in the mild to moderate category.
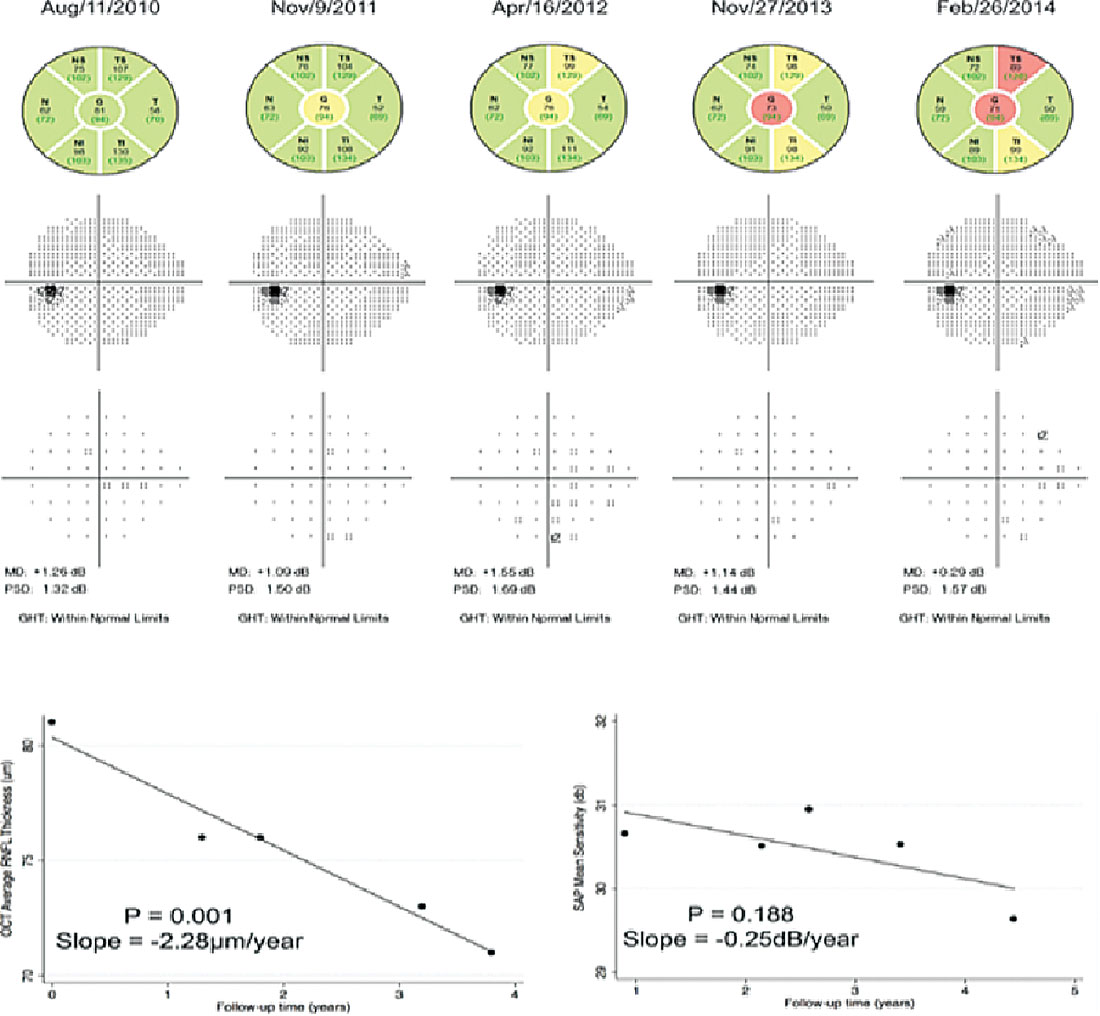 |
| This figure shows the relative stability of serial visual field testing in a patient with mild to moderate glaucoma, whereas OCT shows structural change in the same time period. Click image to enlarge. |
Discussion
With advances in technologies, especially OCT, we’ve grown accustomed to determining change and progression in terms of microns, which has certainly led us to offer better care.
I’ve spoken with many clinicians over the years, and I’m often asked if visual fields will eventually fall by the wayside in glaucoma management since OCT is so precise. Visual fields historically have been difficult tests for patients to undergo, especially the elderly or those with mobility problems. That, coupled with the fact that some patients find field testing to be burdensome and nearly impossible to perform accurately, has led many clinicians to rely less on visual fields and more on OCT imaging to drive the clinical decision-making process.
Fortunately, we’ve seen a new strategy and format for visual fields in glaucoma: virtual reality visual field headsets. I’ve been quite surprised at the level of patient acceptance of these devices. Patients don’t dread the subsequent visit where a visual field is scheduled, nor do I dread telling them we’ll be subjecting them to yet another visual field test. The comfort with which patients can now undergo visual field testing is much greater, but the real question is whether these new field units are valid and reliable. Fortunately, I’ve found them to be just that.
We began using the VisuAll (Olleyes) unit last January. With a large glaucoma practice, I was concerned that incorporating virtual reality technology would interrupt the reliability of information, but I’ve not found that to be the case. Studies have shown the tool’s reliability and comparability to standard HFA visual field testing.1
Backing up, what is the role of visual field testing in managing glaucoma, and will we eventually be able to get rid of it entirely? Right now, I think the answer is clearly no for several reasons.
First and foremost, while OCT technology has resolution in the micron range, there is the undeniable reality that there is a floor effect especially in patients with advanced disease, exactly the ones we should be most concerned about when it comes to progression. As the name implies, as the specific metric being examined begins to erode, whether that is the thickness of the retinal nerve fiber layer or the ganglion cell layer or the minimum rim width of the neuroretinal rim, there is less and less viable tissue remaining. With little tissue remaining, you begin to reach the level of resolution of the OCT instrument itself, making determination of progression more difficult to ascertain.
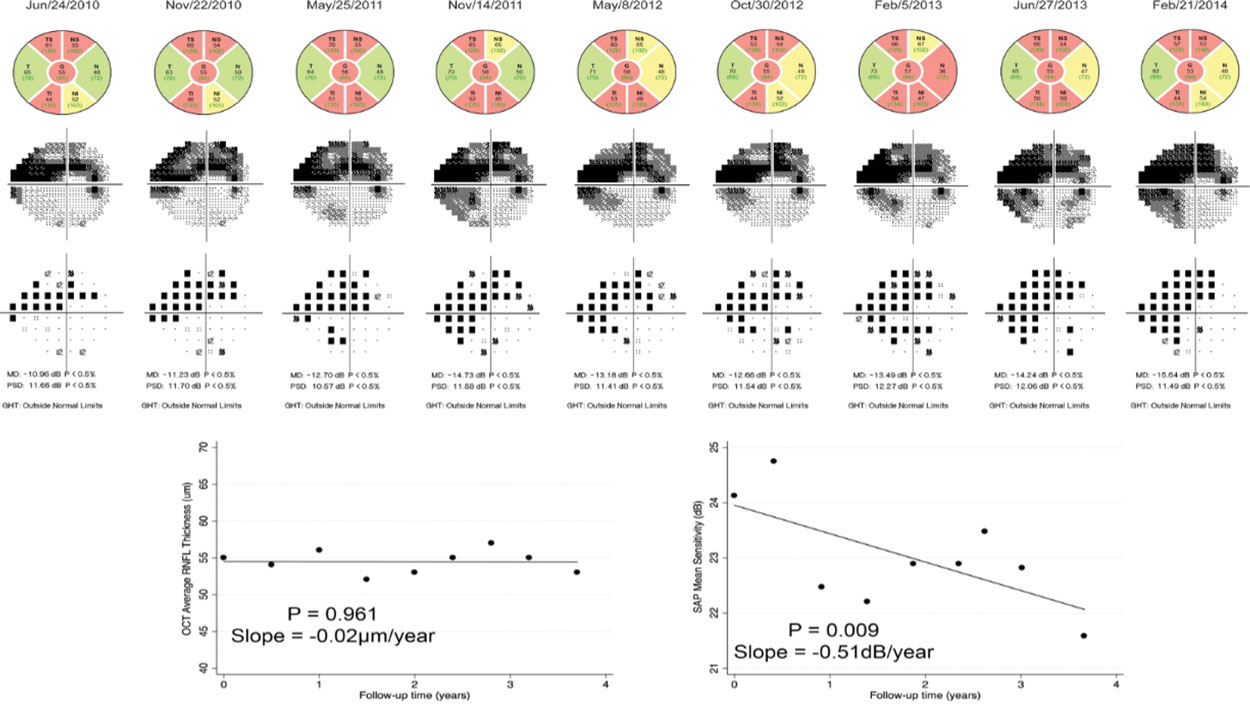 |
This figure shows worsening visual field studies in a patient with more advanced glaucoma over time, whereas OCT does not show comparable change in the same time period. Click image to enlarge. |
Recent studies have looked at the relative odds of glaucomatous progression by structural and functional testing and have found that there is a significant difference depending on the stage of glaucoma.2 In general, in early to moderate glaucoma, progression is more readily seen and identifiable by changes on OCT; whereas in more advanced glaucoma, progression is more readily identified by serial visual field testing. The floor effect may very well be contributing to these results, unsurprisingly. But the reality is that with more advanced disease, we need visual fields to help us determine progression.
What about the other end of the spectrum; namely, patients with early disease? Studies have shown that in early glaucoma, where OCT is employed to identify structural change, visual field testing is critical in identifying concomitant visual field defects. It’s well documented that if we use a 24-2 standard SAP visual field test to determine the presence of field defects in early glaucoma, we will miss defects seen on 10-2 field testing, and those 10-2 defects coincide with the structural changes seen on OCT.3 The 24-2C strategy may help, with its increased number of test points centrally, but 10-2 fields do in fact import valuable information in early glaucoma.
Taking this one step further, what about those patients with ocular hypertension who are at risk of developing glaucoma in the future? If we go back to the Ocular Hypertension Treatment Study (OHTS) study, the data initially showed that the majority of patients who converted from a glaucoma suspect to a glaucoma patient did so due to identifiable structural changes, whereas some showed conversion due to changes in the visual field status, with or without associated structural changes.4 It’s important to note that at the time of the OHTS study, current data pertaining to the advantages of 10-2 threshold field studies in early glaucoma was not well described. That prompts the question of how the prevalence of conversion to frank glaucoma in ocular hypertension would be different if 10-2 fields were employed more often.
Knowing all this, here is my advice to you: proceed as usual, with serial OCTs and visual fields. If you’re not using 10-2 field studies in early glaucoma or patients without visual field defects on a 24-2 strategy, perhaps start doing so. If you’re foregoing visual fields because you are worried that you are overburdening your patients, perhaps invest in a virtual reality field unit. Either way, keep running fields.
Dr. Fanelli is in private practice in North Carolina and is the founder and director of the Cape Fear Eye Institute in Wilmington, NC. He is chairman of the EyeSki Optometric Conference and the CE in Italy/Europe Conference. He is an adjunct faculty member of PCO, Western U and UAB School of Optometry. He is on advisory boards for Heidelberg Engineering and Glaukos.
| 1. Razeghinejad R, Gonzalez-Garcia A, Myers JS, Katz LJ. Preliminary report of a novel virtual reality perimeter compared with standard automated perimetry. J Glaucoma. 2021;30(1):17-23. 2. Abe RY, Diniz-Filho A, Zangwill LM, et al. The relative odds of progressing by structural and functional tests in glaucoma. Invest Ophthalmol Vis Sci. 2016;57(9):421-8. 3. Grillo LM, Wang DL, Ramachandran R, Ehrlich AC, et al. The 24-2 visual field test misses central macular damage confirmed by the 10-2 visual field test and optical coherence tomography. Transl Vis Sci Technol. 2016;5(2):15. 4. Zangwill LM, Weinreb RN, Berry CC, et al. The confocal scanning laser ophthalmoscopy ancillary study to the ocular hypertension treatment study: study design and baseline factors. Am J Ophthalmol. 2004;137(2):219-27. |

