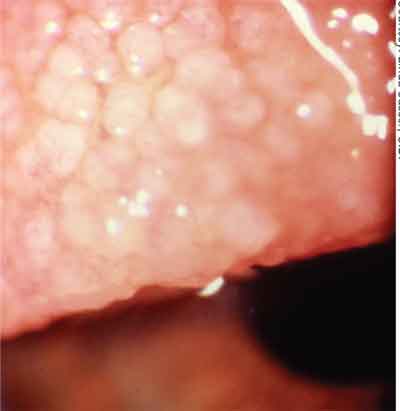
Among the varieties of ocular allergic disease, giant papillary conjunctivitis (GPC) has traditionally been included alongside seasonal allergies and vernal and atopic keratoconjunctivitis. We now know, however, that GPC does not deserve such classification. What we historically thought of as an allergic reaction really is the result of chronic irritation associated with contact lens edges, prostheses, or sutures. Though our knowledge of GPC has advanced since it was first identified, the old categorization lingers.
Besides this incorrect classification, GPC is also stuck with incorrect nomenclature. GPC is neither truly giant nor papillary. In some instances, the condition is referred to as contact lens-induced papillary conjunctivitis, but many eye doctors still call it GPC. And, though it is a conjunctivitis, it might be better designated as a tarsitis. An accurate description would be miniature bumpy upper tarsus.
Here, well discuss how to correctly diagnose and treat this often mislabeled and misunderstood condition.
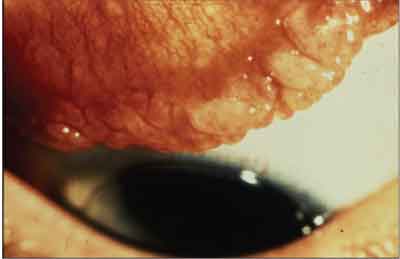
Giant papillary conjunctivitis is not an allergic reaction. Though it is a conjunctivitis, a more accurate description would be miniature bumpy upper tarsus.
The Root of the Problem
GPC occurs when hyperactivity of mast cells and other immune cells promote collagen growth to form conjunctival papillae.1 This growth distinguishes the papillae in GPC from those observed in bacterial conjunctivitis. Although this pathophysiological mechanism can also be found in vernal keratoconjunctivitis (VKC), which represents a true allergic condition, GPC is not an allergic reaction. In ocular allergy, levels of histamine, eosinophils, and eosinophilic major basic protein are elevated; this is not the case with GPC.1
Rather, GPC is a proliferate response to a chronic, physical trauma. The duration and type of stimulus that create this mechanical irritation can also determine the presentation and severity of GPC.
GPC most often originates from contact lens wear. The root of the problem is the lens edge, which rubs against the eye as it blinks 8,000 times per day. This leads to chronic irritation that results in inflammation. The preponderance of GPC in contact lens wearers was originally attributed to an allergy to lens polymer or deposits, contributing to the conditions miscategorization as an allergy.2
|
|
| GPC results from chronic mechanical irritation from a contact lens edge, scleral buckle or suture, which causes inflammation of the tarsal conjunctiva. |
In rare instances, other trauma-causing materialsincluding suture barbs or ocular surface abnormalities such as those caused by extruded scleral buckles or elevated corneal depositsmay also underlie GPC.1
Signs and Symptoms
The characteristic giant papillae of GPC make the condition readily identifiable. Though the disease name implies otherwise, papillae of GPC typically have a diameter no larger than 0.3mm; this is smaller than the >1mm papillae seen in VKC. Papillae in GPC induced by soft lenses typically begin in the upper zone of the tarsal conjunctiva and progress toward the lid margin; they eventually have an evenly dispersed pattern across the tarsal conjunctiva.
Papillae are not as numerous in gas permeable lens wearers. When they do occur, they have a more crater-like shape. The location of the papillae also differs; those induced by GP lenses first form near the lid margin of the tarsal conjunctiva.
The onset of GPC can occur anywhere from a few weeks to a few years after initiating contact lens wear and is typically bilateral, as the cause (contact lens wear) is typically bilateral. GPC may be unilateral if the source of the irritation is not contact lenses, but rather an abnormality, such as a suture, that is also unilateral.
Additional signs and symptoms of GPC include tearing, foreign-body sensation, mild to moderate hyperemia, and stringy or sheet-like mucus production. Itching, a hallmark of true allergic conditions, is generally absent in GPC, and the shield ulcers or other corneal involvement, typical of VKC, do not occur in GPC. Pulling on the upper lid tends to produce significant discomfort for GPC patients due to the inflammatory infiltration in this area.
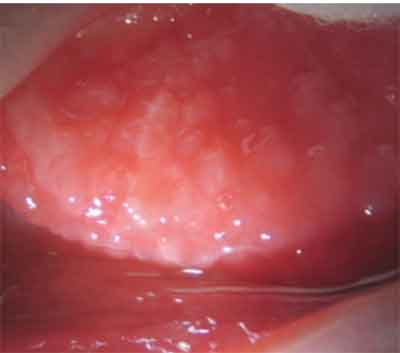 |
| Papillae in GPC are typically less than or equal to 0.3mm in diameter and appear on the upper tarsal conjunctiva. |
GPC can occur simultaneously with other forms of allergy, which makes diagnosis doubly challenging. Patients who have GPC have been documented as having a higher incidence of atopy, namely increased allergies to pollens and to medications.3
Could there be an association between these individuals predisposition to atopy and their development of GPC? The opposite is probably true. Breaks in the ocular surface barrier of GPC patients increase the amount of pollen that can get through to mast cells, which enhances the likelihood of developing ocular allergies. Thus, an ocular allergy and GPC may simultaneously occur in the patient, complicating the diagnosis. The two conditions may each exacerbate the signs and symptoms of the other, worsening the overall condition.
Pathophysiology
The molecular basis of GPC begins with the disruption of normal immune defenses. This stimulates the production and localiza- tion of inflammatory mediators, including neutrophils, eosinophils and mast cells, in the conjunctiva.
Research into the mediators involved in GPC has uncovered more players in the inflammatory processes involved. In a recent study that compared cytokine and chemokine profiles of VKC and GPC, researchers noted four- and eight-fold increases in numerous inflammatory mediators in both diseases respectively.4 Most elevated in GPC were interleukin-6 soluble receptor (IL-6sR), interleukin-11 (IL-11), eotaxin-2, macrophage inflammatory protein (MIP)-1 delta, and tissue inhibitor of metalloproteinase-2 (TIMP-2), although only TIMP-2 and IL-6sR increases were significant when compared with controls. Although some of the same mediators were elevated in both GPC and VKC, TIMP-2 was the only mediator evaluated in this study that was significantly elevated in GPC subjects compared with those who had VKC. IL-6sR was significantly elevated in both VKC and GPC. This, along with the increases in other cytokines, their receptors, and chemokines, emphasizes the importance of these mediators to the pathophysiology of both conditions.
Other research has evaluated the T-cell profiles in GPC and chronic forms of allergy to determine what other inflammatory mechanisms besides a type I hypersensitivity are at work. The shift toward predominately Th2 cytokines was more pronounced in GPC and VKC, whereas atopic keratoconjunctivitis (AKC) revealed a more Th1-like cytokine profile.5 Researchers say these differences may help explain the clinical manifestations of these condi- tions and may influence therapeutic decisions.
Further research revealed that the inflammatory markers human leukocyte antigen (HLA)-DR and intercellular adhesion molecule (ICAM)-1 were elevated above normal in GPC, although the increases were less than those seen in AKC and VKC.6 The elevation of ICAM-1 likely indicates the recruitment of leukocytes by epithelial cells.
Researchers also examined an array of cytokines for their presence or elevation in VKC, AKC and GPC. Some differences were found, such as the upregulation of the cytokine RANTES (Regulated on Activation, Normal T Expressed and Secreted) in GPC but not in AKC and VKC.6 The variability in cytokine profile with each disease may present potential targets for future therapies aimed at each specific condition.
Researchers also revealed that underlying the corneal involvement of VKC and AKC is a greater expression of eosinophil surface antigens than is seen with GPC as well as differences in which cytokines were prominent, as compared with GPC.7
The possible involvement of the protein eotaxin in GPC has gained some interest, yet this remains debatable. Some research shows elevated eotaxin in the tears of contact lens wearers and a correlation between the severity of the signs of GPC with eotaxin levels.8 Yet another study, which evaluated 68 cases of GPC due to ocular prostheses, found no significant differences in the tear eotaxin levels in these individuals compared with healthy subjects.9 In fact, eotaxin levels were diminished in chronic GPC. Further research might establish whether differing etiologies of GPC actually result in distinguishable differences in the profile of inflammatory mediators expressed or whether the temporal progression of GPC from acute to chronic alters eotaxin levels.
Treatment
Research investigating the mediators and pathogenesis of GPC, as well as comparisons of its similarities and differences to chronic allergies, continues. This research holds potential for the development of future therapies.
Management of GPC today, however, is best focused on prevention. Identifying and removing the cause is essential to resolving the condition. Maintain constant alertness for GPC in contact lens wearers. In lens-induced GPC, soft contact lenses are more often implicated than hard lenses.10
Remember that the lens edge is the key component to prevention. We and others have seen cases in which the same lens polymer is used in both eyes, but each has a different lens edge, leading to the development of GPC only in the higher myopic eye. Such cases exemplify the association between GPC and lens edge because all other variables are controlled.
In addition, no one should dismiss proper lens care hygiene and wearing habits. Studies have shown that the frequency of lens replacement correlates closely to incidence of GPC. A study evaluating 47 contact lens wearers found that GPC occurred in 36% of subjects whose lens replacement schedules exceeded four weeks versus 4.5% of those who changed lenses more frequently than once every four weeks.11 While a more frequent lens-replacement schedule cannot entirely eliminate the risk of GPC, the results of this study indicate that this pattern of lens replacement can minimize that risk.
Early identification and removal of the causative factor are the most rapid means by which to resolve GPC. If contact lenses are the cause, removal for one to three weeks typically is sufficient for symptoms to subside, although papillae may linger for months. When contact lens wear resumes, transition the patient to a different type of lens to attempt to find a lens edge design that is less irritating for that individual rather than reintroducing the same lens used at the time of GPC identification.
Steroids such as loteprednol etabonate can be used to treat the inflammation associated with more severe cases of GPC. However, this does not address the underlying cause of the GPC, so their use should be simultaneously accompanied by an intermission of lens wear, and they should not be used on a long-term basis. Mast cell stabilizers may also be prescribed; however, because the mechanism of GPC is not primarily a mast cell-mediated process as seasonal allergic conjunctivitis is, these are of limited efficacy.
By understanding that giant papillary conjunctivitis is not an allergy but an inflammatory condition that results from repeated mechanical irritation (most likely over the contact lens edge), we can better identify and treat these patients. And, the days of GPC masquerading as a true allergy will soon be over.
Dr. Chin is assistant professor of optometry at the New England College of Optometry in Boston. He practices at Andover Eye Associates, Andover, Mass., and performs clinical research at Ophthalmic Research Associates, North Andover, Mass.
1. Greiner JV. Giant Papillary Conjunctivitis. In: Allergic Diseases of the Eye. Abelson MB, ed. New York: WB Saunders; 2001:140-60.
2. Allansmith MR, Korb DR, Greiner JV, et al. Giant papillary conjunctivitis in contact lens wearers. Am J Ophthalmol 1977 May;83(5):697-708.
3. Begley CG, Riggle A, Tuel JA. Association of giant papillary conjunctivitis with seasonal allergies. Optom Vis Sci 1990;67:192.
4. Shoji J, Inada N, Sawa M. Antibody array-generated cytokine profiles of tears of patients with vernal keratoconjunctivitis or giant papillary conjunctivitis. Jpn J Ophthalmol 2006 May-Jun;50(3):195-204.
5. Metz DP, Hingorani M, Calder VL, et al. T-cell cytokines in chronic allergic eye disease. J Allergy Clin Immunol 1997 Dec;100(6 Pt 1):817-24.
6. Hingorani M, Calder VL, Buckley RJ, Lightman SL. The role of conjunctival epithelial cells in chronic ocular allergic disease. Exp Eye Res 1998 Nov;67(5):491-500.
7. Hingorani M, Calder V, Jolly G, et al. Eosinophil surface antigen expression and cytokine production vary in different ocular allergic diseases. J Allergy Clin Immunol 1998 Nov; 102(5):821-30.
8. Moschos M, Eperon S, Guex-Crosier Y. Increased eotaxin in tears of patients wearing contact lenses. Cornea 2004 Nov; 23(8):771-5.
9. Sarac O, Erdener U, Irkec M, et al. Tear eotaxin levels in giant papillary conjunctivitis associated with ocular prostheses. Ocul Immunol Inflamm 2003 Sep;11(3):223-30.
10. Donshik PC. Giant papillary conjunctivitis. Trans Am Ophthalmol Soc 1994;92:687-744.
11. Donshik PC, Porazinski AD. Giant papillary conjunctivitis in frequent replacement contact lens wearers: a retrospective study. Trans Am Ophthalmol Soc 1999;97:205-16.