 |
A 62-year-old Hispanic female presented with gradual progressive central vision loss of both eyes over five years. Her past medical history included rheumatoid arthritis that was controlled with tofacitinib and hypertension that was controlled with lisinopril. She reported drug allergies to codeine and thiopental.
Her best-corrected visual acuity was 20/50 OD and 20/60 OS. Pupils were equally round and reactive to light with no relative afferent pupillary defect, confrontation visual fields were full to finger counting and extraocular motilities were full. IOP was 19mm Hg OD and 17mm Hg OS by applanation. The patient had total color vision deficit by Ishihara color plate testing. Anterior segment examination revealed trace nuclear sclerosis OU. While fundus photos were not obtained, clinical exam revealed a pigmentary bullseye maculopathy OU and macular chorioretinal scar OS. Fundus autofluorescence and OCT imaging are below for review.
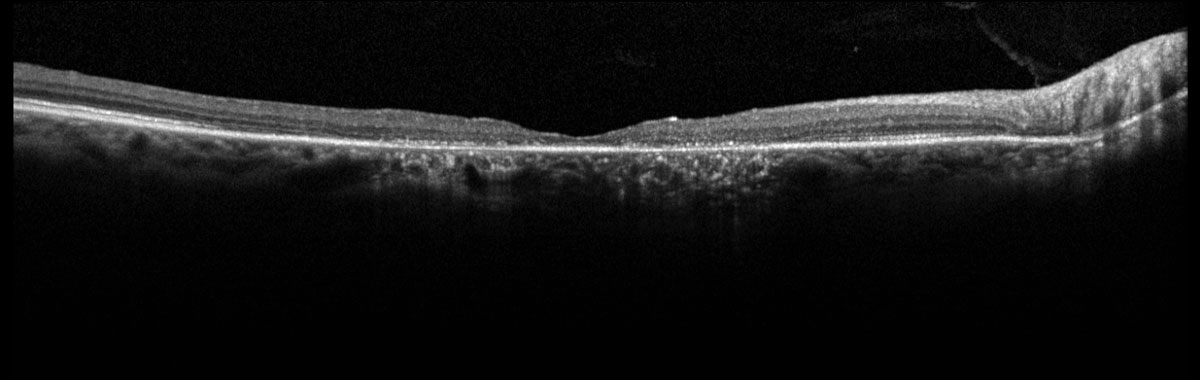 |
Fig. 1. Heidelberg SD-OCT of the right macula. Click image to enlarge. |
 |
|
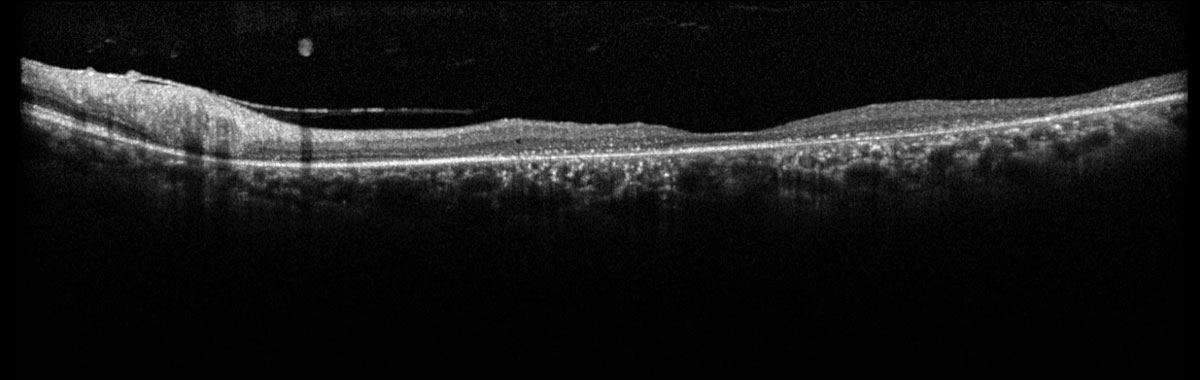
Fig. 2. Heidelberg SD-OCT of the left macula. Click image to enlarge. |
Take the Retina Quiz
1. Which of the following best describes the OCT retinal imaging of both eyes?
a. There is hyperreflectivity of the inner retina.
b. There is perifoveal loss of the inner segment/outer segment junction (IS/OS) and retinal pigment epithelium (RPE).
c. There is subfoveal loss of the IS/OS and RPE.
d. There is subretinal fibrosis.
2. Which of the following is NOT a differential diagnosis for bullseye maculopathy?
a. Cone dystrophy.
b. Hydroxychloroquine toxicity.
c. Presumed ocular histoplasmosis syndrome.
d. Stargardt’s disease.
3. What is the appropriate treatment for this patient’s retinal condition?
a. Broad spectrum oral antibiotics.
b. Cessation of inciting agent and/or observation.
c. Genetic testing.
d. Oral corticosteroids.
4. What is the expected visual prognosis?
a. Complete loss of peripheral vision.
b. Complete resolution to baseline.
c. Progression to no light perception.
d. Stability or slowly progressive decline in central visual acuity.
5. Which of the following is least helpful in detecting early hydroxychloroquine retinal toxicity?
a. Dilated fundus examination.
b. Fundus autofluorescence.
c. Multifocal electroretinogram.
d. Spectral-domain (SD-OCT) of the macula.
Diagnosis
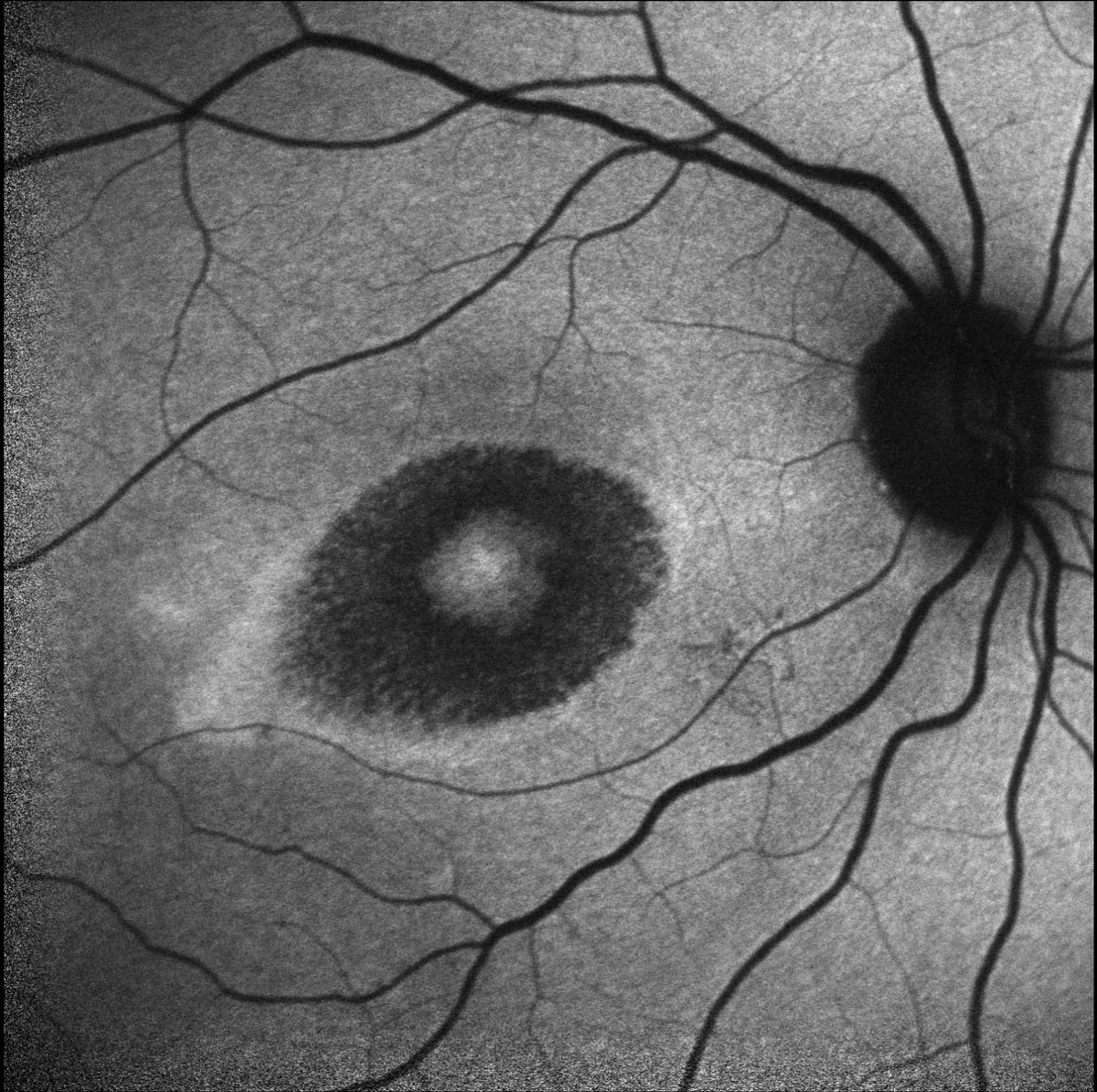
Dilated fundus examination revealed pigmentary changes of the macula in a bullseye pattern in both eyes. Additionally, there was a hyperpigmented chorioretinal scar present in the superonasal macula of the left eye secondary to a reported history of toxoplasma retinochoroiditis 20 years prior. OCT confirmed perifoveal IS/OS and RPE loss, evidenced by perifoveal hypertransmission, with a thin sliver of remaining photoreceptors and RPE underlying the fovea (Figures 1 & 2). Fundus autofluorescence demonstrated perifoveal hypoautofluorescence in both eyes and a dense hypoautofluorescent lesion in the superonasal macula consistent with the chorioretinal scar observed on fundus exam (Figures 3 & 4).
Further questioning revealed that the patient was previously on hydroxychloroquine for 18 years for rheumatoid arthritis. Retinal toxicity was noted six years prior to her presentation to our institute and the hydroxychloroquine was discontinued at that time.
Discussion
The risk of developing retinopathy is most strongly related to daily dose and duration of use.4 The recommended daily dose for prevention of retinal toxicity is less than 5mg/kg of real weight. At this dose, the risk of retinopathy is less than 1% during the first five years of use, less than 2% for 10 years of use and increases to 20% after 20 years of use.4 Other factors that increase risk of ocular toxicity include cumulative lifetime dose greater than 1,000g, renal disease, concomitant tamoxifen use and underlying macular disease.2,4
 |
|
Fig. 3. Heidelberg fundus autofluorescence of the right eye. Click image to enlarge. |
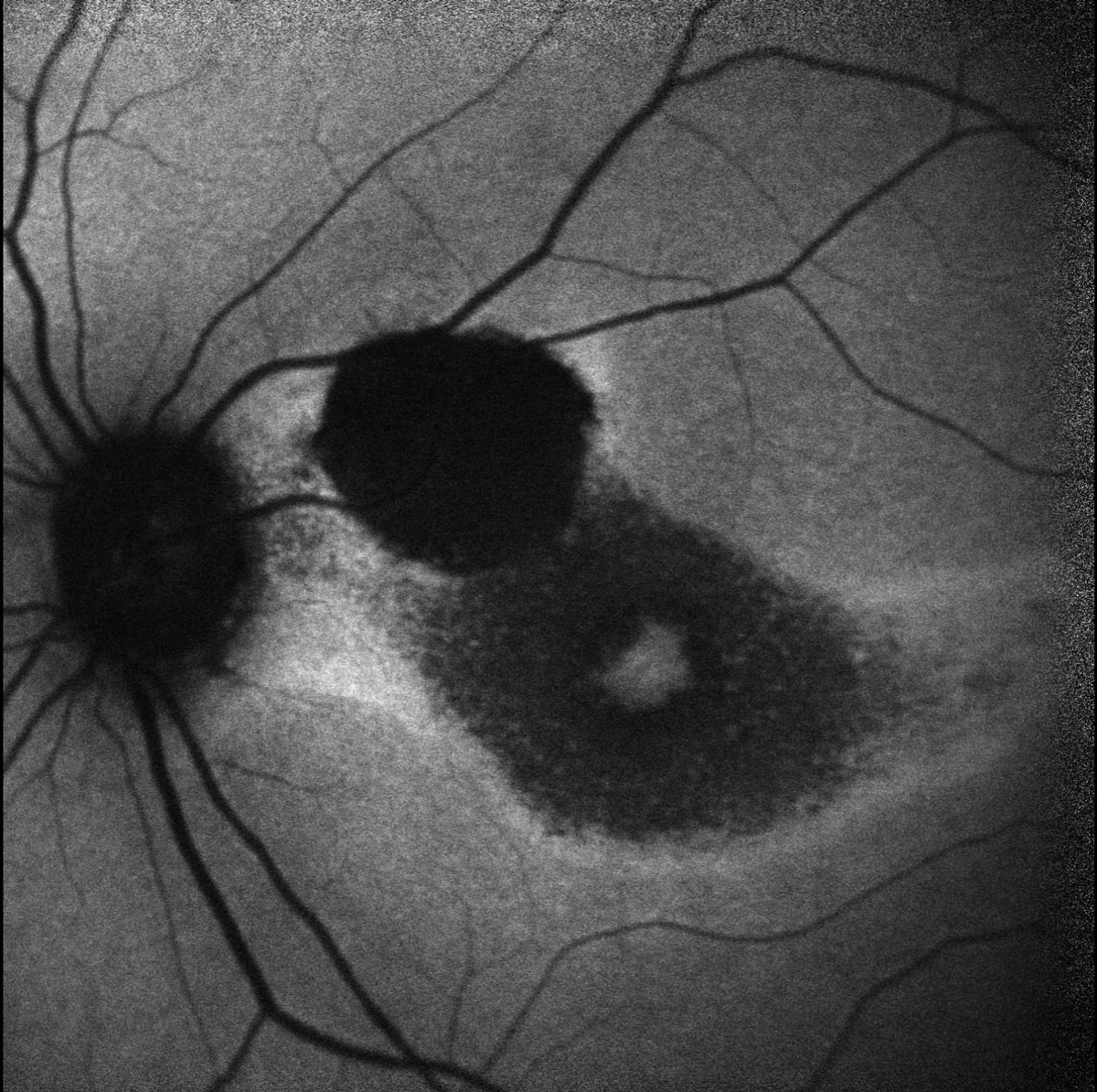 |
Fig. 4. Heidelberg fundus autofluorescence of the left eye. Click image to enlarge. |
Screening and Protection
The American Academy of Ophthalmology updated their screening guidelines in 2016 to include a baseline fundus examination at the time of medication initiation to rule out underlying macular disease, then annual screenings begin after five years.4 Presence of risk factors, including daily dose in excess of the recommendations, may warrant sooner or more frequent screenings.4 Annual screenings should include 10-2 visual field testing for detection of early paracentral depressions and SD-OCT of the macula to look for perifoveal IS/OS.4 The flying saucer sign is an OCT finding characterized by an intact subfoveal IS/OS layer with perifoveal IS/OS loss, creating an appearance of a subfoveal “flying saucer.”5 It is important to note that patients of Asian descent may present with extramacular toxicity, so 30-2 or 24-2 visual fields should be used in addition to 10-2 field testing.4,6
Additional testing can include fundus autofluorescence (bullseye hyperautofluorescence in the acute stage and hypoautofluorescence in the late atrophic stage) and multifocal electroretinography (looking for weak parafoveal responses).4 Previous screening tests such as color vision and Amsler grid testing have fallen out of favor with the advent of more advanced ancillary tests that are more sensitive and specific to detect earlier disease.4
Crucial Communication
Once there is any evidence of retinal toxicity, communication should be initiated with the patient’s rheumatology team informing them of the findings. Ultimately, the decision to continue or stop the drug should be made by the patient and their rheumatologist; however, retinopathy can still progress, even with prompt cessation of the medication due to the slow clearance of the drug and entrapment within the RPE.7 The earlier the retinal toxicity is detected and medication is stopped, the less likely the damage is to progress.1 In patients with minimal RPE atrophy, the retinopathy is likely to stabilize within one year of cessation, but in patients with more advanced RPE damage, progression can continue for as long as 20 years after cessation.7
Optometrists play a key role in screening for retinal toxicity in patients taking hydroxychloroquine and should be well-prepared to detect early changes in order to prevent patients from becoming symptomatic.
Since retinal toxicity is irreversible even after drug cessation, this patient was counseled on the state of their disease and simply observed as there are no further indicated interventions. Communication with the patient’s rheumatology team would have been indicated had she still been on hydroxychloroquine at the time of presentation. This patient’s vision is expected to remain stable or to slowly decline, as there was already extensive perifoveal RPE damage.1
Retina Quiz Answers
1: b, 2: c, 3: b, 4: d, 5: a
Drs. Aboumourad and Leone currently practice at Bascom Palmer Eye Institute in Miami. Neither has any financial disclosures.
Dr. Dunbar is the director of optometric services and optometry residency supervisor at the Bascom Palmer Eye Institute at the University of Miami. He is a founding member of the Optometric Glaucoma Society and the Optometric Retina Society. Dr. Dunbar is a consultant for Carl Zeiss Meditec, Allergan, Regeneron and Genentech.
1. Marmor MF, Hu J. Effect of disease stage on progression of hydroxychloroquine retinopathy. JAMA Ophthalmol. 2014;132(9):1105-12. 2. Ding HJ, Denniston AK, Rao VK, Gordon C. Hydroxychloroquine-related retinal toxicity. Rheumatology. 2015;55(6):957-67. 3. Yam JC, Kwok AK. Ocular toxicity of hydroxychloroquine. Hong Kong Med J. 2006;12(4):294-304. 4. Marmor MF, Kellner U, Lai TYY, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 Revision). Ophthalmology. 2016;123(6):1386-94. 5. Chen E, Brown DM, Benz MS, et al. Spectral domain optical coherence tomography as an effective screening test for hydroxychloroquine retinopathy (the “flying saucer” sign). Clin Ophthalmol. 2010;4:1151-8. 6. Melles RB, Marmor MF. Pericentral retinopathy and racial differences in hydroxychloroquine toxicity. Ophthalmology. 2015;122(1):110-6. 7. Pham BH, Marmor MF. Sequential changes in hydroxychloroquine retiopathy up to 20 years after stopping the drug: implications for mild versus severe toxicity. Retina. 2019;39(3):492-501. |

