 |  |
Most patients seeking to eliminate the need for glasses or contact lenses turn to corneal refractive procedures (LASIK, PRK, SMILE), but high myopes are often best served by a phakic IOL such as Staar Surgical’s Visian posterior chamber implantable collamer lens (ICL).1 The procedure, which is reversible, preserves corneal integrity and accommodative function while correcting up to -16D of myopia.1
Traditionally, the implantation of an ICL requires an iridotomy to allow aqueous flow around the lens and prevent pupillary block complications. Now, patients have the option to have ICL surgery without the need for an iridotomy with the recent FDA approval of Staar’s newest version, called the Evo Visian. This lens has been shown to reduce the incidence of post-surgical complications while maintaining positive visual outcomes equivalent to conventional ICLs.2
 |
|
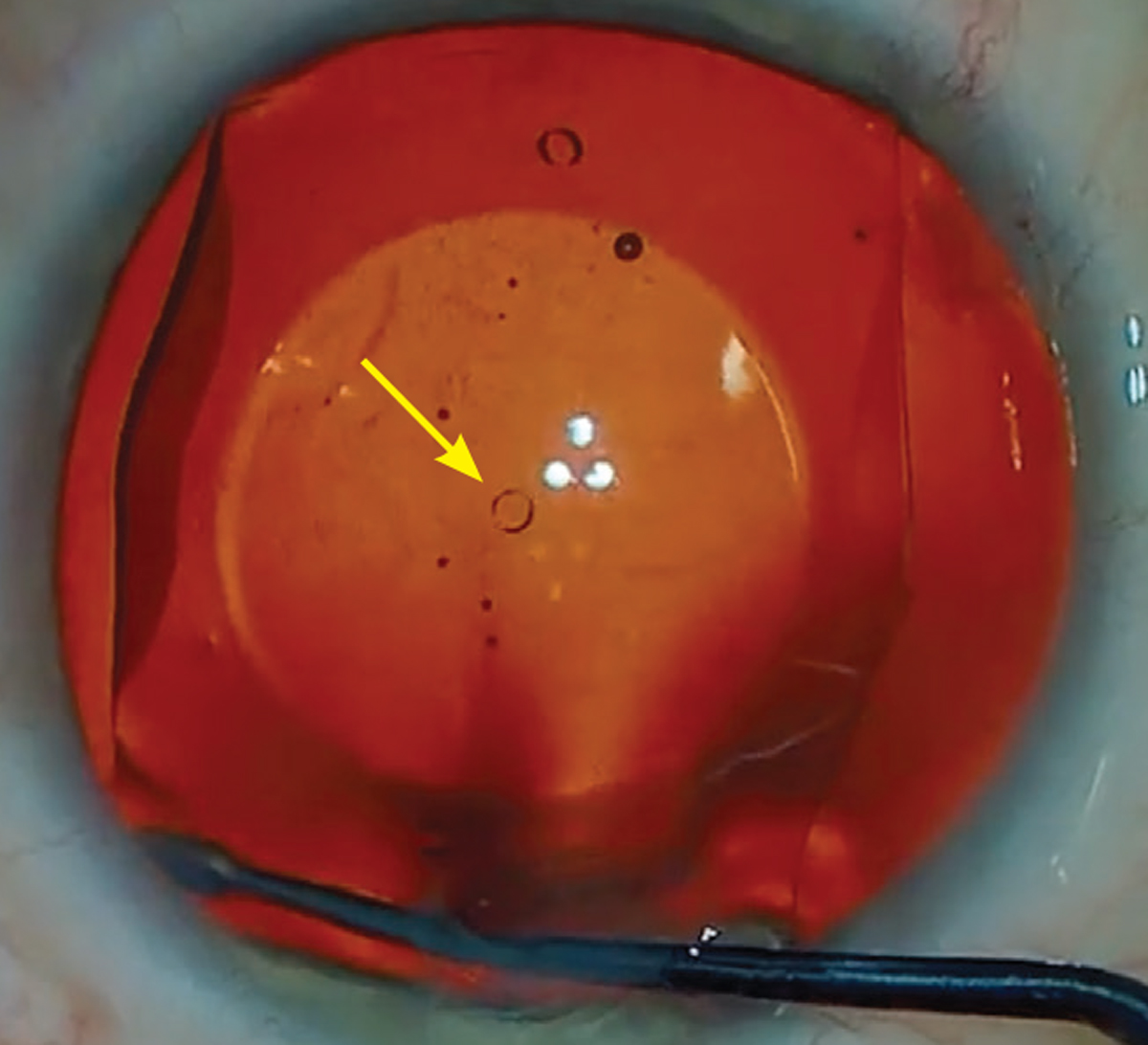
The central port design of the Evo ICL allows for improved circulation of aqueous around the crystalline lens. Photo: Gregory Parkhurst, MD. Click image to enlarge. |
What is EVO ICL?
Like the previous generation Visian, this posterior chamber phakic IOL is made of a biocompatible collagen/polymer (hence, “collamer”) material. However, the new Evo (short for “evolution”) lens features a unique design that includes a 360µm central port.2 This allows sufficient aqueous flow, eliminating the need for the pre-op iridotomy.2 The procedure only takes five to 10 minutes and offers patients a quick visual recovery time. Although the Evo lens has been used around the world for many years, it only recently gained FDA approval; it’s indicated for patients 21 to 45 years of age with a spherical equivalent of -3D to -20D and up to 4D of astigmatism.3 Due to the ICL’s ability to correct large amounts of myopia and astigmatism, it has often been shown to produce postoperative vision that is better than pre-op vision.4
Safety and Effectiveness
The central port design of the Evo ICL allows for improved circulation of aqueous around the natural lens and has reduced the incidence of postoperative increased intraocular pressure and pupillary block.2 In fact, only one case of pupillary block has been reported in 4,196 eyes with over one year of follow-up.2 This design has also resulted in less visually significant anterior subcapsular cataract formation compared to earlier ICL models, due to the efficient fluid dynamics around the anterior lens capsule.2
The question we all want to know is, does this central port impact vision? The current peer-reviewed data shows the port does not significantly alter the optical performance of the Evo lens compared to conventional ICLs, with studies demonstrating equivalent optical quality variables.5 Postoperatively, uncorrected visual acuity averages 20/19, with a range of 20/12 to 20/27.2 Patients with either design report similar instances of initial glare, halos and ring-shaped dysphotopsias at night, with these visual symptoms disappearing over time for up to one year.2
For those with moderate to severe myopia with or without astigmatism, the Evo ICL has demonstrated safety and efficacy in minimizing or eliminating the hassle of corrective lenses. It is a well-established, flexible solution to preserve accommodative function, protect corneal and lens integrity and provide clear vision.
Dr. Groenhuyzen is a refractive surgery and ocular disease resident at Parkhurst Nuvision in San Antonio, TX.
1. Parkhurst GD. A prospective comparison of phakic collamer lenses and wavefront-optimized laser-assisted in situ keratomileusis for correction of myopia. Clin Ophthalmol. 2016;10:1209-15.
2. Packer M. The implantable collamer lens with a central port: review of the literature. Clin Ophthalmol. 2018;12:2427-38.
3. Summary of safety and effectiveness data. FDA. www.accessdata.fda.gov/cdrh_docs/pdf3/P030016S035B.pdf. April 18, 2022. Accessed April 28, 2022.
4. Qin Q, Wu Z, Bao L, et al. Evaluation of visual quality after EVO-ICL implantation for hypermyopia: an observational study. Medicine (Baltimore). 2019;98(44):e17677.
5. Kamiya K, Shimizu K, Saito A, et al. Comparison of optical quality and intraocular scattering after posterior chamber phakic intraocular lens with and without a central hole (hole ICL and conventional ICL) implantation using the double-pass instrument. PLoS One. 2013;8(6):e66846.

