Dig into Dry EyeThis month, Review of Optometry brings you its 24th annual dry eye report. Inside the May issue, multiple ocular surface experts offer their guidance on understanding and managing dry eye in contact lens wearers, the effect of eyelid health and MGD on dry eye and how to detect and treat lacrimal gland abnormalities. Check out the other articles featured in this issue:
|
Preceding the cornea, the tear film is the first surface of the eye that refracts light.1 Altogether, the production, distribution and clearance of the tear film create a highly organized system that maintains ocular surface health.2 Disruption of this process may lead to dry eye disease (DED), causing tear film instability, increased evaporation, inflammation and blurred and fluctuating vision.3
The tear film is composed of an aqueous-mucin layer that contains fluid and soluble factors produced by the lacrimal glands and mucin secreted from goblet cells. Lipid is secreted by the meibomian glands and covers the aqueous-mucin layer. The tear film organization of proteins and glycoproteins functions to maintain a stable, lubricated and smooth optical surface over the cornea. Additionally, the tears contain factors that promote wound healing, suppress inflammation, scavenge free radicals and defend against infection.2
On the eye, contact lenses divide the tear film into a pre-lens and a post-lens component. Identifying current patient tear film and ocular health status allows for proper prescription of lens modality, associated care solutions and topical medications to maintain eye health.
In this article, I’ll review the tear film components, risk factors for DED and considerations for managing dry eye in contact lens wearers. Additionally, I’ll discuss therapeutic lenses currently available for healing severe DED caused by ocular surface disease, as well as touch on some future dry eye-related contact lens technologies in the pipeline.
Regulation of Tear Production
The lacrimal functional unit controls the production, delivery and clearance of tears.3 The components of this unit include the tear-secreting glands (main and accessory lacrimal glands, meibomian glands, conjunctival goblet cells), the surface epithelium, eyelids, lacrimal drainage system, the glandular and mucosal immune system and its connecting innervation.2
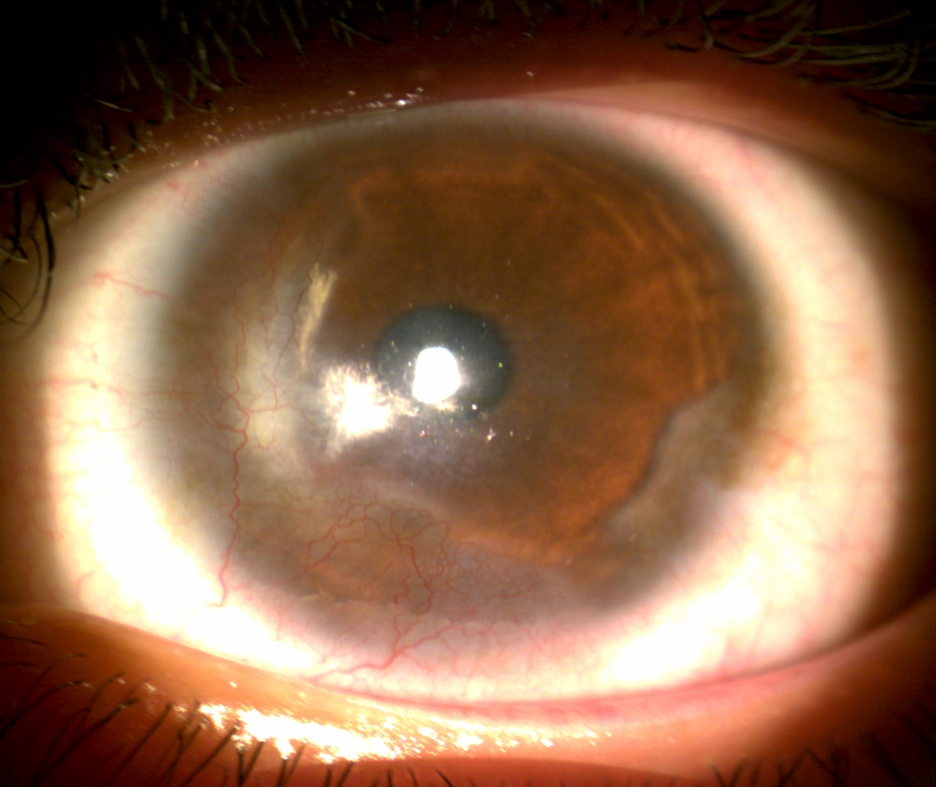 |
| Fig. 1. Limbal stem cell deficiency can develop from DED-related chronic inflammation to the limbus. Click image to enlarge. |
The neural component of the lacrimal functional unit is a reflexive loop starting at the corneal nerves with afferent movement to the central nervous system. This afferent pathway, along with emotional brain centers, connect to secretory and motor efferent nerves that drive tear production and blinking. The efferent pathways end at the accessory lacrimal glands, conjunctival goblet cells and the meibomian glands.
This intricate reflexive loop indicates that secretion of the tear film is tightly controlled to maintain normal homeostasis.2 Damage to the innervation of the lacrimal functional unit, either from surgery or chronic disease, results in decreased tear secretion and surface epithelial barrier disruption. Insult and denervation of the cornea can cause neuropathic pain, including altered tear composition and inflammation.3
One discovered ocular surface nociceptor, TRPM8, has been characterized as the “cold receptor,” as it is stimulated by cooling of the corneal surface between blinks. This cold receptor is thought to have responsibility in driving normal tear flow.4 Interestingly, a new topical ophthalmic medication for evaporative DED is currently being investigated by the FDA that may also act on the TRPM8 receptors.5 Perfluorohexyloctane (F6H8) is a semi-fluorinated alkane liquid that has been used in ocular surgery as a vitreous substitute.6 In former animal studies, F6H8 produced corneal surface temperature changes in tear-deficient guinea pigs.7 It has been suggested that in addition to preventing evaporation, F6H8 may facilitate heat exchange between corneal tissue and the environment, thus reducing corneal temperature and activating TRPM8 cold-thermosensitive channels. In turn, the increased activity of corneal cold nerves may lead to an increase in tear production and blink rate.8
A randomized Chinese clinical trial published this past March showed that topical F6H8 was safe and effective at reducing DED-related symptoms. Additionally, in this population, the drop was well-tolerated and increased tear film break-up time and lipid layer thickness.5
Currently, F6H8 is commercially available in Europe for the treatment of evaporative DED. In the US, a formulation of F6H8 manufactured and commercialized by Bausch + Lomb had its New Drug Application accepted by the FDA in September 2022. This ophthalmic topical medication has a PDUFA action date of June 28, 2023.
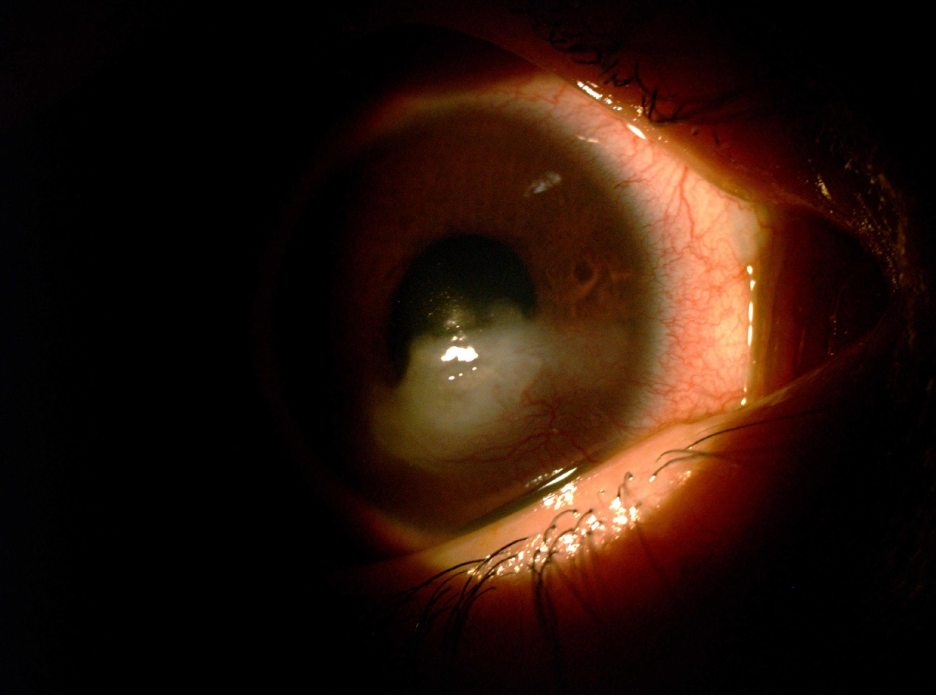 |
| Fig. 2. Exposure keratitis can cause severe dryness and desiccation in the cornea, causing scarring. Click image to enlarge. |
Tear Film Components
To better understand the ways in which dry eye and contact lens wear intersect, let’s review the functions of the primary tear film components, including mucins, lipids and the aqueous-mucin tear layer.
Mucins. Tear mucus is composed of water and mucin glycoproteins that serve to maintain barrier function, hydration and wettability of the hydrophobic surface epithelial cell membranes. Also, the tear mucus provides a matrix for lacrimal secreted factors and minimizes friction from blinking. The cornea and conjunctival epithelial cells produce membrane-associated mucins that are major components of the wettable glycocalyx.9 In addition, the goblet cells secrete a gel mucus that moves over the ocular surface, which also contributes to tear stability by binding water.10
Goblet cell loss occurs in systemic inflammatory diseases, such as Sjögren’s syndrome, Stevens-Johnson syndrome and graft-vs.-host disease.11 Eyes with significant goblet cell loss due to Stevens-Johnson syndrome or Sjögren’s syndrome are at risk for developing sight-threatening corneal ulceration and opacification.12
Lipids. The surface lipid layer of the tear film is primarily derived from the meibomian glands and serves as the interface between the aqueous layer and the air. The lipid layer functions as a smooth optical surface and reduces surface tension of the tear film. Furthermore, the lipid layer prevents anterior migration of aqueous tears from the lid margin and retards evaporation.13 During a blink, the lipid layer is first compressed towards the lower lid, then spreads upward as the lid opens. Altered spreading and thinning of the lipid layer in those with meibomian gland disease contributes to tear film instability.14
Other tear film components. The aqueous-mucin tear layer contains numerous proteins, including growth and supportive factors. Some of these have a homeostatic function (e.g., suppress inflammation and maintain innervation), while others participate primarily in epithelial and/or stromal wound healing.15 For example, anti-infective molecules, including lysozyme, lactoferrin and lipocalin, have been found in the normal tear film.16,17 The basic antibacterial mechanism of these factors involves their ability to bind free iron necessary for bacterial growth.18 Also, there are several antioxidants, including ascorbic acid and cysteine, that scavenge and protect the ocular surface against damage from free radicals.19
Demographic and Lifestyle Risk Factors
DED is associated with several demographic and lifestyle factors. Patients of female sex and individuals with reduced sleep duration are at risk of aqueous-deficient DED. Additionally, increased screen exposure is risk factor for evaporative dry eye. Advancing age and elevated psychological stress may also be associated with both aqueous-deficient and evaporative DED.20
Being female is an independent risk factor for the development of aqueous-deficient dry eye disease. This association has been hypothesized to be mediated by hormone regulatory actions of the hypothalamic-pituitary axis, sex-specific steroids and the thyroid gland. Additionally, the complex interactions between the immune system, autonomic pathways and the lacrimal functional unit may play a role in this predisposition in female patients.20
Inadequate sleep is associated with increased risk of aqueous-deficient DED. Although the mechanism is not well understood, there is a positive association between DED and sleep disorders, decreased sleep duration and sleep quality. Poor sleep duration and quality reduce production of androgens and decreases parasympathetic tone. These preceding events lead to the downregulation of tear secretion from the lacrimal glands. Moreover, sleep deprivation may alter the circadian patterns of the hypothalamic-pituitary-adrenal axis and renin-angiotensin-aldosterone system, leading to excessive diuresis, natriuresis and dehydration, which might also impact aqueous tear production.20
Increased digital device screen exposure was identified to be a risk factor for the development of evaporative dry eye. Device use may suppress spontaneous and reflex blinking during tasks involving significant levels of cognition and visual processing. The decrease in blink rate and completeness diminishes the delivery of meibum to the ocular surface, thereby increasing the risk of evaporative DED.20
Advancing age is associated with increased risk of aqueous-deficient and evaporative DED. Dry eye is an age-related degenerative condition that progresses with lifetime cumulative exposure to a diverse range of physiological and environmental factors, as well as hormonal changes, which can all lead to ocular surface inflammation and neurosensory abnormalities. Additionally, mental health disorders, such as anxiety and depression, increase psychological stress. This mechanism may exacerbate pre-existing ocular surface homeostatic disturbances through the modulation of immune, hormonal and neurosensory systems.20
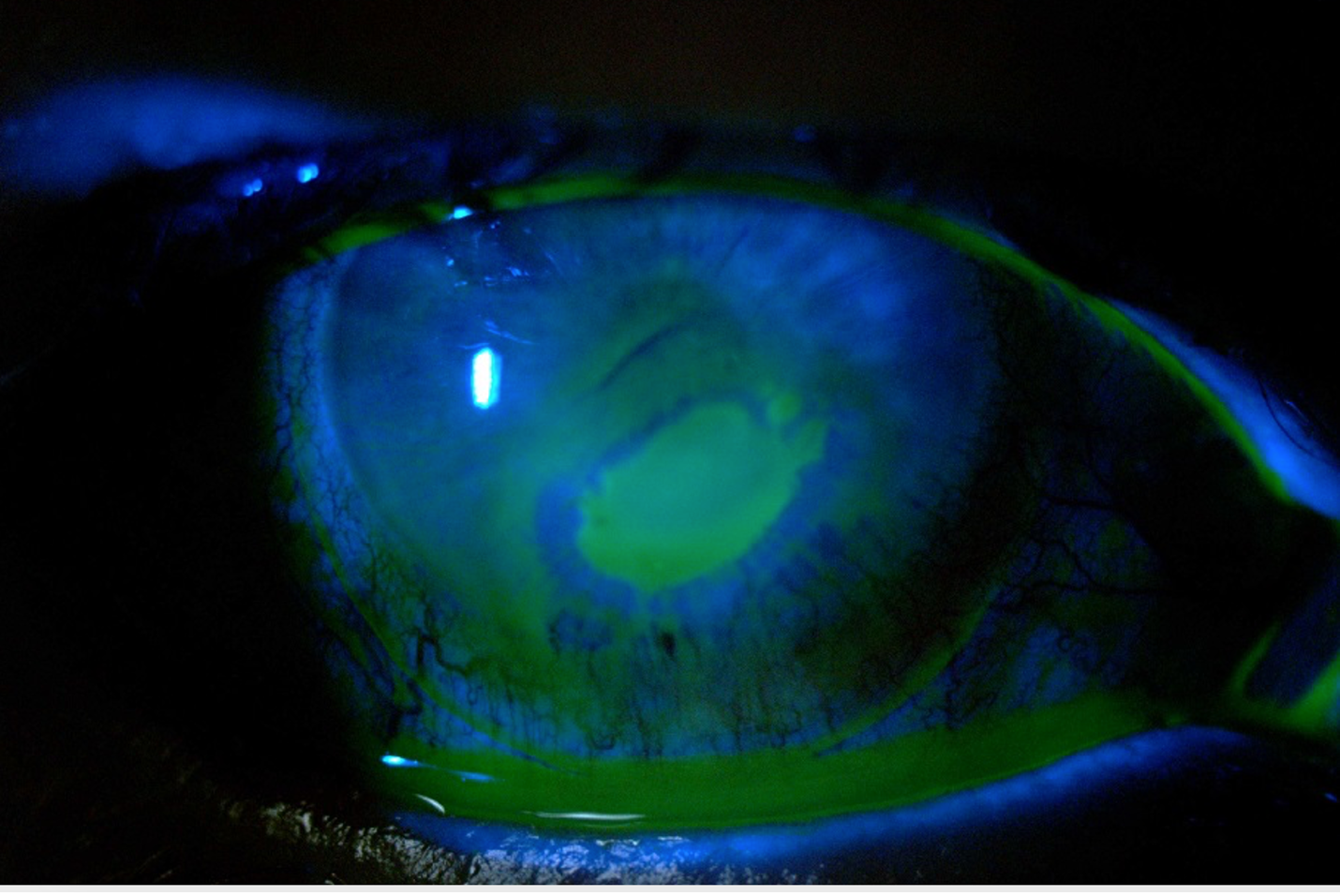 |
| Fig. 3. Persistent epithelial defects can occur from loss of corneal innervation from dry eye. Click image to enlarge. |
Tear Film and Contact Lenses
The tear film is a critical component of the eyes' optical system. The tears and the anterior surface of the cornea account for approximately 80% of the eye’s refractive power.21 Deterioration in corneal surface smoothness causes reduced contrast sensitivity and increased optical aberrations that degrade retina image quality.22 As already mentioned, destabilization of the tear film due to external or local factors upsets the delicate homeostatic balance at the ocular surface and gives rise to DED. When a contact lens is placed on eye, it divides the tear film. This alteration of the precorneal tear film may be a risk factor for DED.23
Contact lens use was reported as a risk factor for dry eye in several large epidemiological studies on DED.23,24 The interaction of contact lens with the tear film results in an increased rate of evaporation, reduced tear thickness and volume, delayed spreading of the lipid layer, reduced pH, increased tear ferning, increased osmolarity of the pre-lens tear film and increased friction between the lens and surface epithelium, all of which can cause uncomfortable DED symptoms.25 Additionally, several lens-induced ocular changes have been associated with the etiology of contact lens-related dry eye, including decreased goblet cell density and alterations in mucin produced by goblet cells.26,27 Also, changes to meibomian gland function increasing the number of plugged and expressible orifices as well as gland dropout have both been associated with lens wear.28
Ocular discomfort due to dryness has been identified in a number of studies as a primary reason for the discontinuation of contact lenses.29 Inflammation is a core mechanism of DED, its damage to the ocular surface and the sensation of discomfort. Several inflammatory markers, including interleukins, were increased in tears those with contact lens-related dry eye. These results support a growing evidence that suggests a potential role of inflammation in contact lens-related dry eye.30
Lens-Related DED Treatments
Options for managing this type of dry eye include daily disposable lenses, topical artificial tears, hydroxypropyl cellulose ophthalmic inserts, omega-3 and omega-6 fatty acids, punctal plugs and azithromycin.31-36 In addition, studies have shown that both topical lifitegrast and cyclosporine ophthalmic solution may be safe and effective therapeutic interventions for managing patients with contact lens–related dry eye.34,37
Let’s dive into a few therapeutic options for contact lens wearers experiencing dry eye, followed by a sneak peek at some prospective management options in the pipeline.
Medical lenses for severe dry eye. Individuals with severe DED from ocular surface diseases such as limbal stem cell deficiency (Figure 1), neurotrophic keratitis, exposure keratitis (Figure 2) and persistent epithelial defects (Figure 3) can benefit from therapeutic scleral lenses. The unique design of a scleral lens vaults the cornea while landing on the sclera conjunctival shape. A fluid reservoir filled typically with non-preserved sterile saline constantly hydrates the cornea, thus facilitating its healing process and preventing further desiccation of the ocular surface.38
Topical medications, such as antibiotics, can be added into the vault of the lens before insertion to aid surface therapy.39 Further promoting healing, the large-diameter lens protects the ocular surface from shearing forces of the eyelids. Also, the gas permeable lens design corrects irregular astigmatism from corneal abnormalities.38
Lens care considerations. Contact lens care systems have important implications for lens-wearing comfort.40 In addition to providing exceptional disinfection and cleaning, the fundamental features of hydrogen peroxide (H2O2) lens care systems can also help promote lens wear comfort. One difference between H2O2 and multipurpose solutions is that the former systems are preservative-free. For lens wearers with sensitivity to preservatives, including some patients with dry eye or contact lens-associated dryness symptoms, H2O2 will help avoid discomfort associated with preservative sensitivity.41
In a small randomized study of symptomatic lens wearers, the use of a one-step H2O2 system resulted in significantly greater lens wettability than the use of polyhexamethylene biguanide-containing multipurpose solutions.42 Also, an analysis of multiple lens and lens care system combinations found that participants using a one-step H2O2 system had significantly greater subjective comfort ratings than multipurpose solution users upon lens insertion.43
Surface wetting agents can improve the comfort of contact lenses in those experiencing dry symptoms. Recently, a novel covalently bonded polyethylene glycol (PEG)-based lens surface treatment was designed to improve lens wettability, deposit resistance and tear break-up time to enhance contact lens comfort. A double-masked crossover study found that PEG surface-treated scleral lenses provided improved comfort and reduced both DED symptoms and ocular surface compromise compared with untreated participants with DED. Additionally, PEG-based surface treatment improved contact lens comfort in soft and gas-permeable contact lens wearers.44
Future contact lens technologies. Topical ophthalmic medications are part of a mainstay treatment for those with contact lens-related dry eye and ocular surface disease. Topical medications are rapidly removed by the nasolacrimal duct and clearance of the tear film, as well as by conjunctival blood and lymphatic flow.45 In fact, only 1% to 5% of the administered topical medication is absorbed by the tissue.46 To counter low bioavailability, frequent instillation of medication is needed, which impacts patient compliance.
Instead of topical medications, drug-eluting contact lenses may be an alternative treatment for eye conditions such as DED. Drug delivery with therapeutic lenses increases the time of the medication in front of the cornea, therefore, increasing bioavailability to approximately 50%.47 Additionally, this modality of drug delivery may encourage treatment compliance due to the elimination of multiple drops throughout the day. Drug-eluting soft lenses loaded with cyclosporine and dexamethasone have been investigated for DED and corneal inflammation, respectively.48,49
Takeaways
The ocular surface is a privileged but delicate environment. Disruptions in the homeostasis of the ocular surface can impact visual perception, induce pain states and generally degrade quality of life.
A stable tear film is critical to maintaining a healthy ocular surface. This surprisingly complex emulsion is susceptible to disruption from a variety of insults, including idiopathic disease, injury, surgery, infection and contact lenses. DED commonly results from altered tear film functioning and can be exacerbated by or even caused by contact lenses. At the same time, considered use of contact lenses can also ameliorate the signs and symptoms of dry eye.
Looking ahead, foreseeable advances in contact lens technology may improve the ability of contact lenses to heal the ocular surface and also to prevent dry eye’s development.
Dr. Frogozo is the owner of Alamo Eye Care in San Antonio, Texas, where she directs the Contact Lens Institute. She is also a fellow of the American Academy of Optometry and the Scleral Lens Education Society, an advisory board member of the Gas Permeable Lens Institute, a committee member of the Contact Lens and Cornea Section of the American Optometric Association, an associate member of the International Society of Contact Lens Specialists and a member of the Texas Optometric Association. She discloses financial relationships with Boston Sight, LenTechs, CooperVision and Euclid.
1. Tutt R, Bradley A, Begley C, Thibos LN. Optical and visual impact of tear break-up in human eyes. Invest Ophthalmol Vis Sci. 2000;41(13):4117-23. 2. Pflugfelder SC, Stern ME. Biological functions of tear film. Exp Eye Res. 2020;197:108115. 3. Rosenthal P, Borsook D. Ocular neuropathic pain. Br J Ophthalmol. 2016;100(1):128-34. 4. Parra A, Madrid R, Echevarria D, et al. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat Med. 2010;16(12):1396-99. 5. Tian L, Gao Z, Zhu L, et al. perfluorohexyloctane eye drops for dry eye disease associated with meibomian gland dysfunction in chinese patients: a randomized clinical trial. JAMA Ophthalmol. 2023;141(4):385-92. 6. Zeana D, Becker J, Kuckelkorn R, Kirchhof B. Perfluorohexyloctane as a long-term vitreous tamponade in the experimental animal. Experimental perfluorohexyloctane substitution. Int Ophthalmol. 1999;23(1):17-24. 7. Acosta MC, Luna C, Quirce S, Gallar J. Infrared thermography of the ocular surface of tear-deficient eyes treated with perfluorohexyloctane. Invest Ophthalmol Vis Science. 2017;58:465. 8. Delicado-Miralles M, Velasco E, Díaz-Tahoces A, et al. Deciphering the action of perfluorohexyloctane eye drops to reduce ocular discomfort and pain. Front Med (Lausanne). 2021;8:709712. 9. Gipson IK, Spurr-Michaud S, Tisdale A, Menon BB. Comparison of the transmembrane mucins MUC1 and MUC16 in epithelial barrier function. PLoS One. 2014;9(6):e100393. 10. Gipson IK, Argüeso P. Role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol. 2003;231:1-49. 11. Wang Y, Ogawa Y, Dogru M, et al. Baseline profiles of ocular surface and tear dynamics after allogeneic hematopoietic stem cell transplantation in patients with or without chronic GVHD-related dry eye. Bone Marrow Transplant. 2010;45(6):1077-83. 12. Bagga B, Motukupally SR, Mohamed A. Microbial keratitis in Stevens-Johnson syndrome: Clinical and microbiological profile. Ocul Surf. 2018;16(4):454-7. 13. Georgiev GA, Eftimov P, Yokoi N. Structure-function relationship of tear film lipid layer: A contemporary perspective. Exp Eye Res. 2017;163:17-28. 14. Braun RJ, King-Smith PE, Begley CG, Li L, Gewecke NR. Dynamics and function of the tear film in relation to the blink cycle. Prog Retin Eye Res. 2015;45:132-64. 15. Klenkler B, Sheardown H, Jones L. Growth factors in the tear film: role in tissue maintenance, wound healing, and ocular pathology. Ocul Surf. 2007;5(3):228-39. 16. Wiesner J, Vilcinskas A. Antimicrobial peptides: the ancient arm of the human immune system. Virulence. 2010;1(5):440-64. 17. Dartt DA. Tear lipocalin: structure and function. Ocul Surf. 2011;9(3):126-38. 18. Flanagan JL, Willcox MD. Role of lactoferrin in the tear film. Biochimie. 2009;91(1):35-43. 19. Ohashi Y, Dogru M, Tsubota K. Laboratory findings in tear fluid analysis. Clin Chim Acta. 2006;369(1):17-28. 20. Wolffsohn JS, Wang MTM, Vidal-Rohr M, et al. Demographic and lifestyle risk factors of dry eye disease subtypes: a cross-sectional study. Ocul Surf. 2021;21:58-63. 21. Rolando M, Zierhut M. The ocular surface and tear film and their dysfunction in dry eye disease. Surv Ophthalmol. 2001;45 Suppl 2:S203-10. 22. Rieger G. The importance of the precorneal tear film for the quality of optical imaging. Br J Ophthalmol. 1992;76(3):157-58. 23. Gomes JAP, Azar DT, Baudouin C, et al. TFOS DEWS II iatrogenic report. Ocul Surf. 2017;15(3):511-38. 24. Doughty MJ, Fonn D, Richter D, et al. A patient questionnaire approach to estimating the prevalence of dry eye symptoms in patients presenting to optometric practices across Canada. Optom Vis Sci. 1997;74(8):624-31. 25. Craig JP, Willcox MD, Argüeso P, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the contact lens interactions with the tear film subcommittee. Invest Ophthalmol Vis Sci. 2013;54(11):TFOS123-56. 26. Colorado LH, Alzahrani Y, Prithard N, Efron N. time course of changes in goblet cell density in symptomatic and asymptomatic contact lens wearers [published correction appears in Invest Ophthalmol Vis Sci. 2016;57(8):3891]. Invest Ophthalmol Vis Sci. 2016;57(6):2888-94. 27. Berry M, Pult H, Purslow C, Murphy PJ. Mucins and ocular signs in symptomatic and asymptomatic contact lens wear. Optom Vis Sci. 2008;85(10):E930-8. 28. Alghamdi WM, Markoulli M, Holden BA, Papas EB. Impact of duration of contact lens wear on the structure and function of the meibomian glands. Ophthalmic Physiol Opt. 2016;36(2):120-31. 29. Richdale K, Sinnott LT, Skadahl E, Nichols JJ. Frequency of and factors associated with contact lens dissatisfaction and discontinuation. Cornea. 2007;26(2):168-74. 30. Ramamoorthy P, Khanal S, J Nichols J. Inflammatory proteins associated with contact lens-related dry eye. Cont Lens Anterior Eye. 2022;45(3):101442. 31. Lazon de la Jara P, Papas E, Diec J, Naduvilath T, Willcox MD, Holden BA. Effect of lens care systems on the clinical performance of a contact lens. Optom Vis Sci. 2013;90(4):344-50. 32. Sindt CW, Longmuir RA. Contact lens strategies for the patient with dry eye. Ocul Surf. 2007;5(4):294-307. 33. Luchs JI, Nelinson DS, Macy JI; LAC-07-01 Study Group. Efficacy of hydroxypropyl cellulose ophthalmic inserts (LACRISERT) in subsets of patients with dry eye syndrome: findings from a patient registry. Cornea. 2010;29(12):1417-27. 34. Wang L, Chen X, Hao J, Yang L. Proper balance of omega-3 and omega-6 fatty acid supplements with topical cyclosporine attenuated contact lens-related dry eye syndrome. Inflammopharmacology. 2016;24(6):389-96. 35. Li M, Wang J, Shen M, et al. Effect of punctal occlusion on tear menisci in symptomatic contact lens wearers. Cornea. 2012;31(9):1014-22. 36. Nichols JJ, Bickle KM, Zink RC, Schiewe MD, Haque RM, Nichols KK. Safety and efficacy of topical azithromycin ophthalmic solution 1.0% in the treatment of contact lens-related dry eye. Eye Contact Lens. 2012;38(2):73-79. 37. Gonzalez AL. Safety and efficacy of lifitegrast 5% ophthalmic solution in contact lens discomfort. Clin Ophthalmol. 2018;12:2079-85. 38. Harthan JS, Shorter E. Therapeutic uses of scleral contact lenses for ocular surface disease: patient selection and special considerations. Clin Optom (Auckl). 2018;10:65-74. 39. He X, Donaldson KE, Perez VL, Sotomayor P. Case series: overnight wear of scleral lens for persistent epithelial defects. Optom Vis Sci. 2018;95(1):70-5. 40. Jones L, Brennan NA, González-Méijome J, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the contact lens materials, design, and care subcommittee. Invest Ophthalmol Vis Sci. 2013;54(11):TFOS37-70. 41. Carnt N, Stapleton F. Silicone hydrogel lens-solution interaction and inflammation. Eye Contact Lens. 2013;39(1):37-41. 42. Guillon M, Maissa C, Wong S, Patel T, Garofalo R. Effect of lens care system on silicone hydrogel contact lens wettability. Cont Lens Anterior Eye. 2015;38(6):435-41. 43. Diec J, Papas E, Naduvilath T, Xu P, Holden BA, Lazon de la Jara P. Combined effect of comfort and adverse events on contact lens performance. Optom Vis Sci. 2013;90(7):674-81. 44. Mickles CV, Harthan JS, Barnett M. Assessment of a novel lens surface treatment for scleral lens wearers with dry eye. Eye Contact Lens. 2021;47(5):308-13. 45. Janagam DR, Wu L, Lowe TL. Nanoparticles for drug delivery to the anterior segment of the eye. Adv Drug Deliv Rev. 2017;122:31-64. 46. Novack GD. Ophthalmic drug delivery: development and regulatory considerations. Clin Pharmacol Ther. 2009;85(5):539-43. 47. Bengani L, Chauhan A. Are contact lenses the solution for effective ophthalmic drug delivery? Future Med Chem. 2012;4(17):2141-3. 48. Kim J, Mondal H, Jin R, et al. Cellulose acetate phthalate-based ph-responsive cyclosporine a-loaded contact lens for the treatment of dry eye. Int J Mol Sci. 2023;24(3):2361. 49. Bengani LC, Kobashi H, Ross AE, et al. Steroid-eluting contact lenses for corneal and intraocular inflammation. Acta Biomater. 2020;116:149-61. |


