Focus on RetinaJune's issue is our 13th annual retina report, which explores the latest in clinical research and innovation in the detection and management of retinal diseases. Check out the other featured articles here: |
Age-related macular degeneration (AMD) is the third leading cause of blindness worldwide and the leading cause of legal blindness among people aged 65 years and older in the United States.1-3 It is estimated that approximately half of all causes of severe vision loss (20/200 or worse) in US individuals living over the age of 40 are caused by AMD.4,5 Only about 10% to 20% of individuals with AMD have the exudative form of the disease, the advanced condition accounts for the majority—90%—of AMD-related severe central vision loss.3,4,6
AMD is a commonly encountered disease, especially in developed countries. In pooled data from the US, Netherlands and Australia, the prevalence of AMD was 4.6% among individuals aged 75 to 84 years and 13.1% among individuals over the age of 84 years. This same study found that neovascular AMD was present in 0.17% of individuals aged 55 to 64 years and 5.8% for those older than 85.7,8 Due to a growing proportion of older adults in the US the prevalence of AMD is expected to rise, and the number of cases of advanced AMD is estimated to increase from 1.7 million in 2010 to 3.8 million in 2050.4,7,9
AMD is an acquired degenerative macular disease usually affecting individuals over the age of 55 years. It is characterized by pathologic alterations of the outer retina, retinal pigment epithelium (RPE), Bruch’s membrane and choriocapillaris complex including drusen formation and pigmentary changes.1,3,7 AMD is a progressive disease, and in advanced stages, central geography atrophy and neovascularization—either choroidal or subretinal—may develop and reduce vision.3
Neovascular AMD is caused by proliferation of new and abnormal blood vessels, usually originating from the choriocapillaris, that invade the subRPE and/or subretinal space through a disrupted Bruch’s membrane.1,7,10 Neovascular membranes may be exudative or non-exudative. These fragile abnormal vessels lack a proper inner blood-retina barrier and may leak out fluid containing protein and lipids or blood.3,7,11 When leakage of fluid or blood occurs and a neovascular complex is found, the term exudative neovascular AMD is most appropriate.
Interestingly, few cases of non-neovascular, yet exudative, AMD have been reported, where intraretinal fluid was found without evidence of neovascularization or retinal vascular abnormalities to account for such. Like neovascular exudative AMD, non-neovascular exudative AMD also appears responsive to anti-VEGF therapy.12
Early detection of exudative AMD is of utmost importance so that treatment may be instituted without delay and vision can be saved.
 |
|
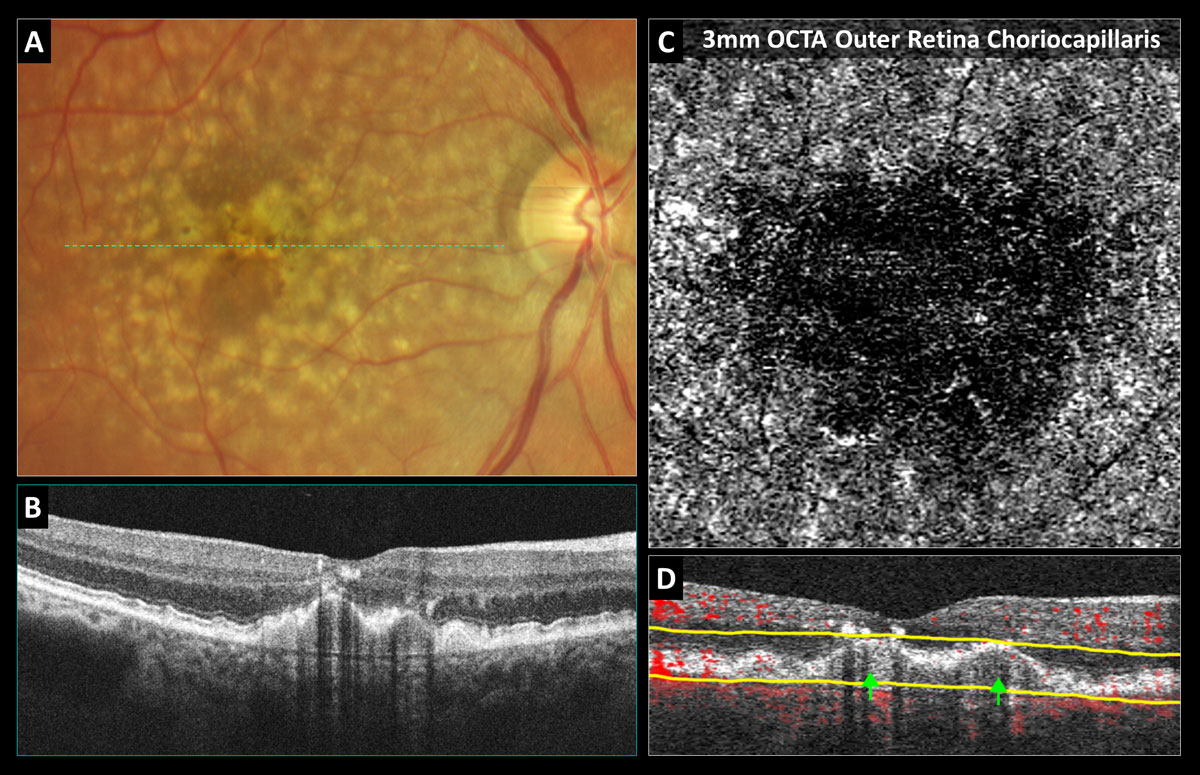
Fig. 1. OCT-A confirms avascularity of a drusenoid pigment epithelial detachment (PED): (A) Color photo shows soft coalesced drusen with overlying hyperpigmentary changes. (B) Structural OCT reveals a PED with medium uniform internal reflectivity, no fluid and shadowing artifacts from the overlying intraretinal pigmentary migration. (C) 3mm OCT-A outer retina choriocapillaris enface display shows choriocapillaris nonperfusion without neovascularization. (D) OCT-A B-scan overlay with yellow segmentation boundaries reveals no red blood flow overlay internally within the PED. Click image to enlarge. |
 |
|
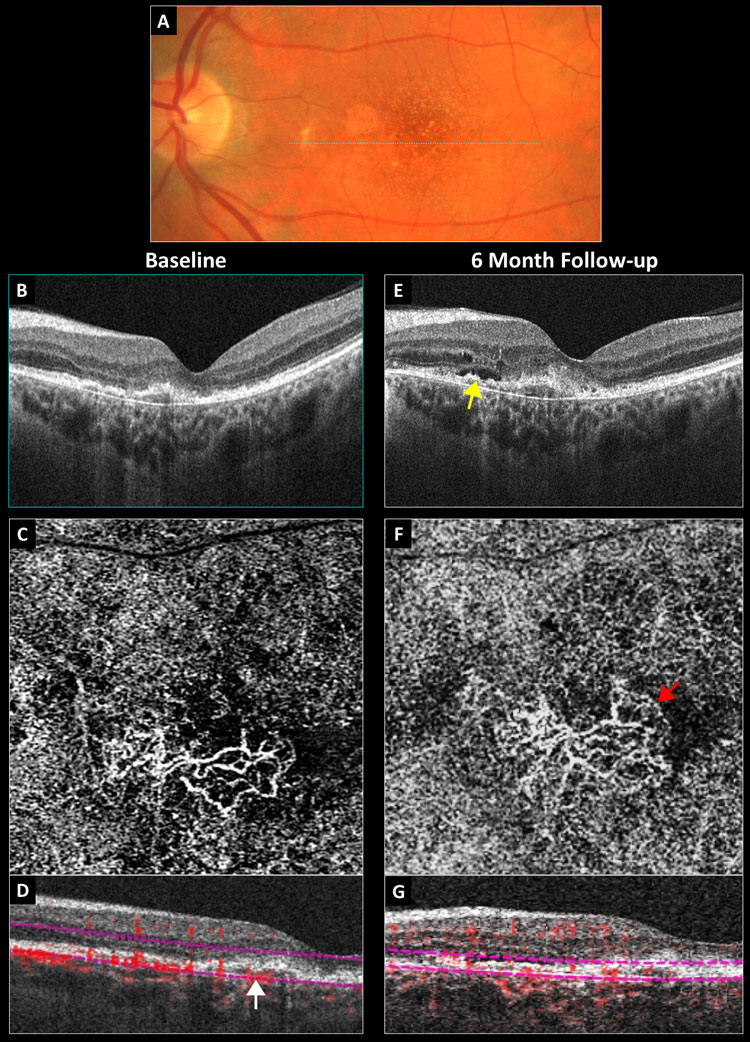
Fig. 2. Conversion of a non-exudative neovascular membrane to exudative AMD: (A) baseline color photograph, (B) baseline structural OCT, (C) baseline 3mm OCT-A outer retina choriocapillaris en-face display, (D) baseline OCT-A B-scan overlay with the white arrow pointing to a vascularized PED, (E) six-month structural OCT with the yellow arrow pointing to new subretinal fluid, (F) six-month 3mm OCT-A with the red arrow pointing to a peripheral fringe of lacy capillaries and (G) six-month OCT-A B-scan overlay. Click image to enlarge. |
Step 1: Identify Eyes at High-risk
We know that not all patients with AMD will convert to the exudative and highly vision-threatening form of the disease. Therefore, it is important to be familiar with high-risk clinical features so that individuals at high-risk for conversion can be identified. Patients that exhibit these high-risk AMD phenotypes should be monitored in-office more frequently and educated on symptoms of conversion and provided with self-screening tools. Recommending dietary and lifestyle modifications, such as smoking cessation, as well as vitamin supplementation when appropriate, may also reduce the risk of exudative conversion.3
Fundus findings that increase the risk for exudative conversion include large-sized drusen (especially soft subtype), as well as RPE clumping and pigment migration anteriorly into the retina (Figure 1A).7 Eyes with intermediate non-exudative AMD, or AREDS category 3, are at greater risk for conversion to advanced AMD, which occurs in approximately 18% of cases within five years, compared to those with early stage non-exudative AMD.4,13 Intermediate stage dry AMD is defined as the presence of either (1) extensive medium-sized drusen (63-124 µm in diameter), (2) at least one large sized druse (≥125 µm in diameter) or (3) non-central geographic atrophy.4,13 Among patients that already have neovascular AMD in the fellow eye, the risk for neovascularization in the eye with non-exudative AMD is very high, approximately 42% at five years.7,14
There are certain drusen subtypes that pose a greater risk for future neovascularization including soft drusen and subretinal drusenoid deposits, also referred to as reticular pseudodrusen. With OCT, soft drusen appear as round, mound-shaped elevations of the RPE from Bruch’s membrane that are filled internally with drusenoid material that is homogenous (uniformly reflective) and relatively dark or hypo-reflective (Figure 1B). Subretinal drusenoid deposits are characterized on OCT as nodular, hyper-reflective deposits that rest on the anterior surface of the RPE. Research has shown that eyes with a combination of both soft drusen and subretinal drusenoid deposits are at highest risk for conversion to neovascular AMD.15 Although more research is needed, lower macular pigment optical density and impaired dark adaptation may also pose an increased risk for AMD progression.16,17
The American Optometric Association Clinical Practice Guidelines advise that patients with soft confluent drusen and granular pigmentary degeneration be monitored at four to six month intervals.3 Similarly, the American Academy of Ophthalmology Preferred Practice Patterns recommend that “patients at exceptionally high-risk (e.g., the presence of advanced AMD in one eye and large drusen with RPE changes in the fellow eye) may be examined more frequently (i.e., every six to 12 months) in an effort to detect asymptomatic CNV at a treatable stage.”4
With the advent of OCT angiography (OCT-A) we now know that non-exudative neovascular membranes exist in eyes with AMD and is an entity that poses a greater risk for future exudative conversion.18-20 Neovascular AMD and exudative AMD are not synonymous terms, and the presence of exudation is the main factor in determining whether anti-VEGF therapy is indicated.
A non-exudative neovascular membrane is characterized by the presence of a well-defined neovascular complex via OCT-A in a treatment naïve eye that has no signs of exudation via ophthalmoscopy, such as exudate or hemorrhage, and no fluid on structural OCT imaging.18-20 This vascular anomaly is present in approximately 10% of eyes with intermediate AMD are already exudative in the fellow eye.18-20
Non-exudative neovascularization carries with it a substantial risk for conversion to the exudative form of the disease, and research suggests that the risk of conversion to exudative AMD in eyes with nonexudative choroidal neovascularization (CNV) is about 15 times greater than those without.18-20 Although most retina specialists are not treating non-exudative neovascular membranes, there may be value in referring to them, especially if the membrane is identified in the better seeing eye of a monocular patient.4,18-20
Figure 2 depicts the left eye of an asymptomatic 68-year-old previously diagnosed with dry AMD bilaterally. Baseline exam revealed best-corrected acuity of 20/40 in the left eye. Color photography revealed macular pigmentary changes and noncentral geographic atrophy (Figure 2A). Structural OCT showed an irregular and shallow PED with medium internal hyperreflectivity and no fluid in the nasal fovea (Figure 2B). The 3mm OCT-A outer retina choriocapillaris enface display revealed a well-defined sub-RPE neovascular membrane with adjacent choriocapillaris nonperfusion (Figure 2C). On the corresponding OCTA B-scan overlay, the PED appeared internally vascularized and was red (Figure 2D). The patient was diagnosed with a non-exudative neovascular membrane in the left eye and told to follow-up in three months.
He returned six months later reporting stable vision. Acuity and ophthalmoscopy were unchanged; however, repeat structural OCT revealed new subretinal and intraretinal fluid overlying the PED (Figure 2E). Repeat OCT-A showed that the neovascular membrane was slightly larger with a peripheral fringe of lacy capillaries morphologically suggestive of exudative activity (Figure 2F). The patient was referred to a retinal specialist and treated with anti-VEGF.
 |
|
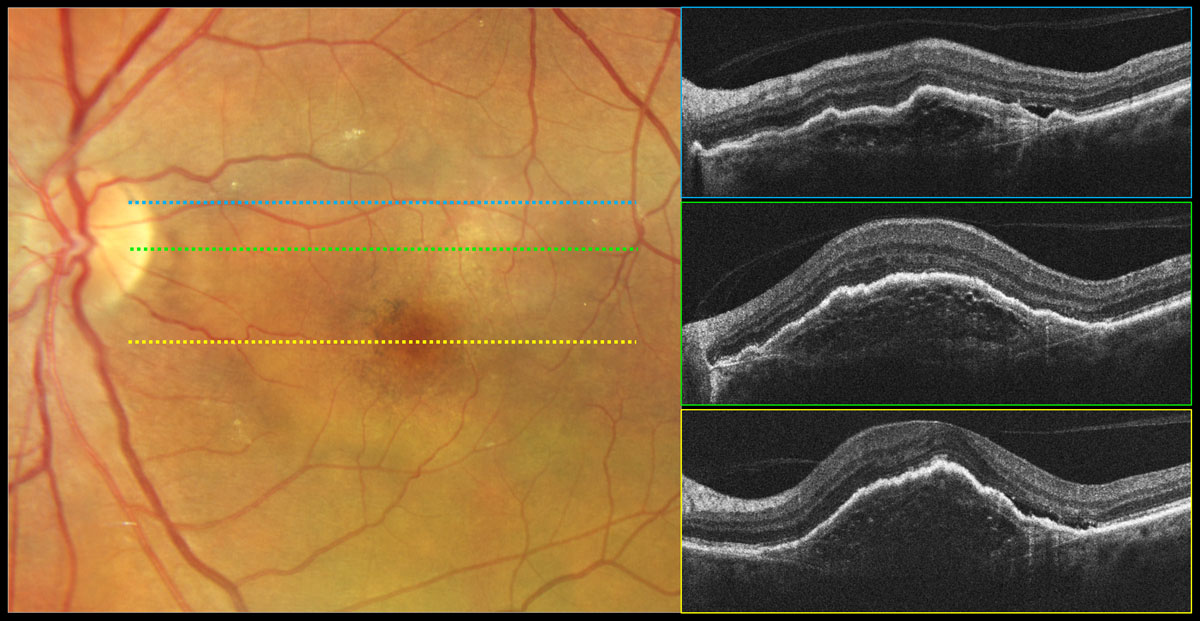
Fig. 3. Type 1 neovascular exudative AMD, fibrovascular PED. Color photography shows a round lighter area of the macula that is elevated corresponding to a fibrovascular PED with overlying pigmentary changes. OCT reveals a PED with irregular surface contour and variable (nonhomogeneous) internal reflectivity containing multilaminate hyperreflective sheets. Bruch’s membrane can be visualized as a thin hyperreflective line at the base of the PED. Subretinal fluid is present on the temporal aspect of the PED likely due to exudation vs. mechanical stress. Click image to enlarge. |
Step 2: Recognize Symptoms of Exudative Conversion
It is important to educate all patients with AMD, as well as your office staff, regarding symptoms of exudative conversion and the need for prompt examination, typically within 24 hours of onset, when they occur. Symptoms of dry-to-wet conversion tend to develop rather rapidly over the course of days to weeks and are more severe and unilateral in nature compared to those caused by progression of non-exudative AMD.1,3,7 They may include painless and new-onset metamorphopsia, loss or blurring of central vision, and sudden development of a new central scotoma (positive scotomas that a patent is aware of may be caused by hemorrhage).4,7,10 Micropsia may also occur in the setting of serous macular detachment.10At home self-screening is strongly encouraged, and patients should be provided with an Amsler grid and near acuity chart, as well as educated on how to properly use these screening tools. Consider mobile applications and prescription devices for those at particularly high-risk for conversion, such as those with intermediate stage non-exudative AMD who still have useful vision in at least one eye.
Providing the patient with written information on symptoms suggestive of exudative conversion and educating the patient’s loved ones not may be of particular benefit since AMD affects the elderly who may also suffer from memory loss. Home self-screening tools do not replace the need for frequent in-office examinations, since patients may be asymptomatic at the time of exudative conversion.4 This is an ideal time to catch and treat exudation so that vision can be preserved.
 |
|
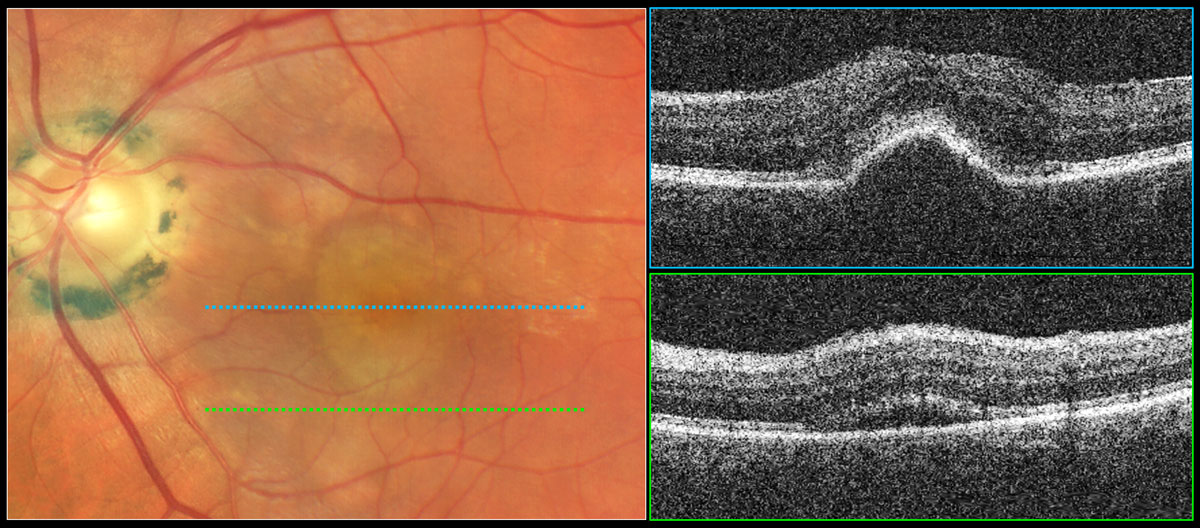
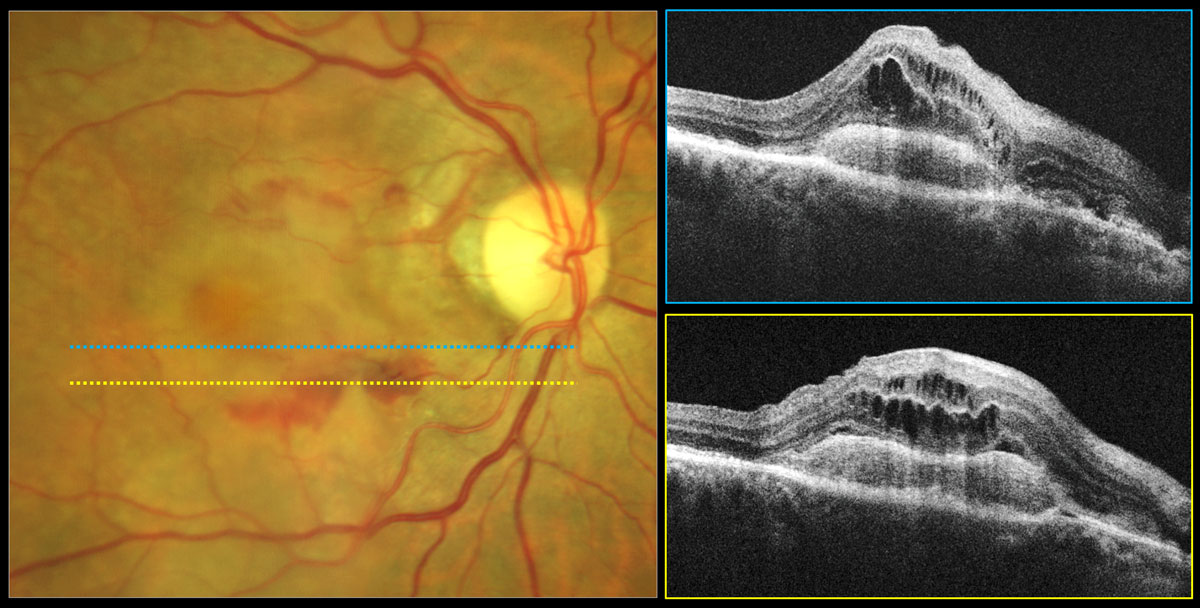
Fig. 4. Serous PED with subretinal fluid suggestive of exudative AMD. Color photography shows a well-demarcated, dome-shaped, yellowish elevation of the RPE in the central macula. The top OCT raster scan reveals elevation of the RPE with a rather smooth surface and dark internal reflectivity consistent with a serous PED. The bottom OCT raster scan reveals subretinal fluid suggestive of exudation from a neovascular source requiring treatment. Click image to enlarge. |
 |
|
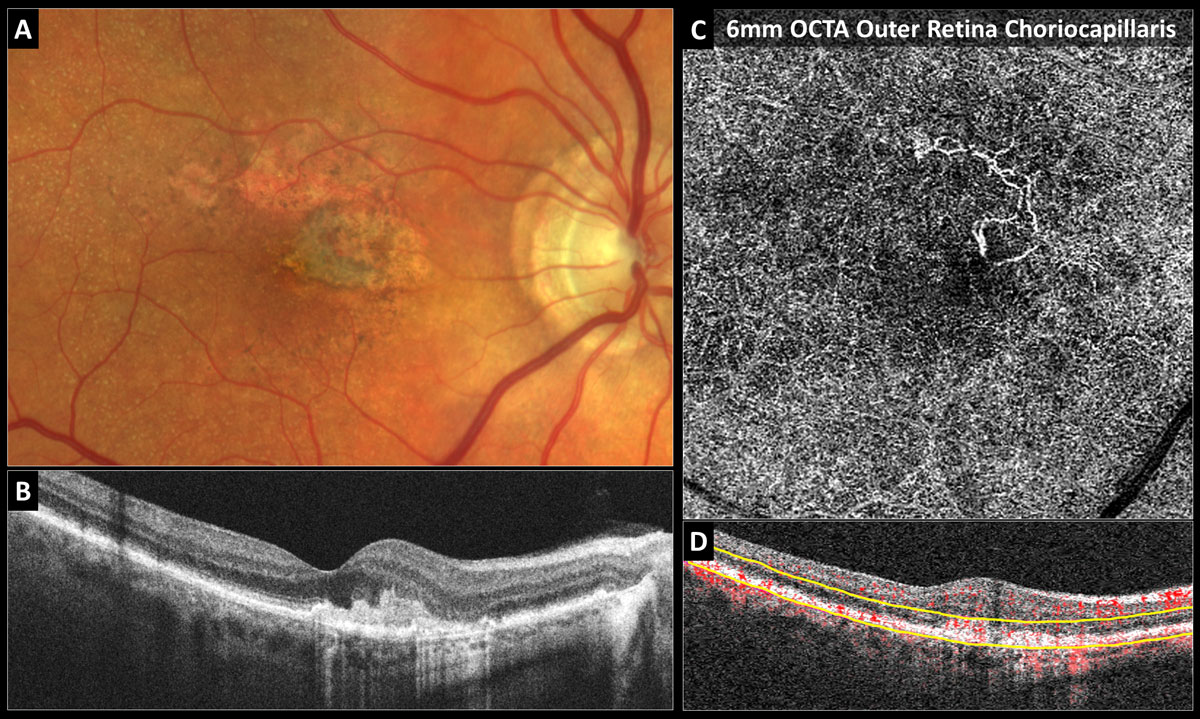
Fig. 5. Inactive type 2 neovascular AMD: (A) Color photo shows the gray-green subretinal fibrovascular complex in the nasal fovea with adjacent RPE atrophy and reticular pseudodrusen. (B) Structural OCT reveals a subretinal hyperreflective fibrovascular complex and no fluid. (C) The 6mm OCT-A outer retina choriocapillaris en-face display shows inactive long filamentous neovascular vessels. (D) OCT-A B-scan overlay with yellow segmentation boundaries. Click image to enlarge. |
Step 3: Perform a Thorough Fundus Exam
In those previously diagnosed with AMD or new patients complaining of central vision loss and/or metamorphopsia, dilated slit lamp biomicroscopy should be performed using a low powered noncontact pre-corneal lens, such as a 60D lens, that enhances stereopsis. Every attempt should be made to maximize stereopsis during biomicroscopy, especially when you don’t have access to OCT.
The central portion of the contact Goldmann 3-mirror is also of benefit in detecting subtle outer retinal thickening but should be performed after ancillary imaging tests are completed. It is very important to note that OCT does not replace the need for fundus examination since subtle hemorrhages, suggestive of exudation and a need for treatment, are often invisible on OCT.
The fundus and OCT features of active wet AMD occur because of fluid or blood leakage. Fluid or blood commonly accumulate in the sub-RPE, subretinal and intraretinal spaces, but preretinal and vitreous hemorrhage are less common features of exudative AMD that have been reported.3 The clinical manifestations of neovascular and/or exudative AMD include the following:
Hemorrhage. As previously mentioned, hemorrhage from exudative AMD can occur at any depth location; it may be sub-RPE (resulting in a hemorrhagic PED), subretinal, intraretinal and less commonly preretinal or vitreous.3 Biomiscroscopy and fundus photography are superior to OCT in the detection of hemorrhage, which is a relatively common manifestation of neovascular AMD that is suggestive of active exudation.4 Recall that the retina itself is translucent; therefore, a small, shallow subretinal hemorrhage will appear brightly red on fundus exam (Figures 6 and 7). On the other hand, a large collection of subretinal blood will be darker red. Subretinal hemorrhages are redder and brighter compared to the dark green sub-RPE hemorrhages, which are masked by RPE pigment.1 Visualizing unobscured retinal blood vessels anterior to the hemorrhage can help differentiate subretinal hemorrhages from nerve fiber layer and preretinal ones.
Pigment epithelial detachment (PED). This is defined as a separation of the RPE from Bruch’s membrane and is a common finding in both dry and wet AMD forms.11 There are various types of PEDs: fibrovascular, hemorrhagic, serous and drusenoid. Use of multimodal imaging—OCT, OCT-A, fluorescein angiography (IVFA) and indocyanine green angiography (ICG)—is beneficial and sometimes necessary to differentiate various types of PED.11
Drusenoid PEDs are associated with intermediate stage dry AMD and formed by coalesced soft drusen, while hemorrhagic and fibrovascular PEDs are always associated with exudative and/or neovascular AMD. Clinically drusenoid PEDs appear as yellow-white areas of RPE elevation with possibly scalloped borders and intralesional spots of hyperpigmentation (Figure 1A).
Fibrovascular PEDs result from a type 1 neovascularization complex that proliferates between Bruch’s membrane and the RPE. Clinically, they manifest as a mostly opaque area of RPE elevation often with an irregular surface contour that may contain a mix of blood, fibrotic tissue and fluid (Figure 3).1,11
Hemorrhagic PEDs appear as round or oval-shaped dark-green colored elevations of the RPE with discrete borders.1,3
Serous PEDs result from serous fluid accumulation between the RPE and Bruch’s membrane. Clinically they appear as well-demarcated, dome-shaped, yellowish-orange elevations of the RPE (Figure 4).3 While serous PEDs may be a manifestation of non-exudative AMD due to compromise of the outer blood-retinal barrier, they are more often a manifestation of exudative AMD and should greatly raise suspicion for the presence of neovascularization until proven otherwise.11,21 When a serous PED is found, multimodal imaging, often including IVFA and ICG, must be performed to look for a treatable neovascular source.11
Grey-green subretinal/sub-RPE thickening. Occasionally, and especially in cases of subretinal (type 2) neovascularization, the neovascular complex itself may be visualized with biomicroscopy as a grey-green or pinkish-yellow plaque-like membrane (Figure 5A).1,7, 10 When a neovascular complex is exudative, which is often the case, the area of grey-green thickening may be surrounded by hemorrhage, exudate and/or fluid (Figure 6).10
Exudate. Fluid that leaks out from incompetent neovascular vessels contains high density lipoproteins, which may deposit and become trapped within the retina (intraretinal exudate) or under the retina (subretinal exudate).1,10 Exudate alone, without fluid, does not indicate that a neovascular membrane is currently exudative and requires treatment. The amount of exudate may actually increase with successful anti-VEGF therapy and will likely remain for a lifetime. Clinically, it can be difficult to differentiate drusen from exudate since both tend to be yellow-white in coloration. Drusen are located deeper in the retina while exudate exuded from leaky neovascularization is often more superficial and located intraretinally or subretinally.1 Drusen also tend to be more diffusely distributed throughout the macula and posterior pole, while exudate tends to be more localized within clusters.22 When in doubt, OCT can be very valuable in depth localizing an unknown yellow-white lesion.
Fibrovascular disciform scar and RPE tear. Subretinal/sub-RPE fibrosis and scarring as well as an RPE tear are also manifestations of exudative AMD found in later stages of the disease.3,11
Step 4: Use Multimodal Imaging
Incorporating techniques such as color fundus photography, fundus autofluorescence (FAF), OCT, OCT and IVFA/ICG (when appropriate and within scope) can greatly benefit your evaluation of eyes with AMD.OCT. This is generally considered standard of care in the evaluation of AMD and can greatly facilitate the clinician’s ability to detect the earliest neovascular and exudative AMD possible, refer for prompt treatment and prevent future vision loss. It is also of great value in monitoring for exudative recurrence and serves as guide during anti-VEGF therapy.
The sensitivity for neovascularization detection with structural OCT alone is excellent and is even better when used in combination with OCT-A.4,23 It is important when reviewing OCT data to scan through the entire cube, looking for fluid and RPE elevation, rather than just one central raster B-scan. Before detailing structural OCT features suggestive of exudation, understand the OCT classification system of choroidal CNV that divides into various subtypes depending upon the anatomic location of the membrane itself.
Type 1 (occult). This type of CNV membrane is in the sub-RPE space between Bruch’s membrane and the RPE and is the most common type of CNV present in neovascular AMD, found in 80% of cases. They are often consistent with occult leakage patterns (late leakage of an undetermined source) on conventional IVFA.4,10 Via structural OCT, type 1 CNV manifests as a fibrovascular PED (Figures 2E and 3). On OCT-A they appear as an ill-defined network of vessels and are usually larger membranes with mature feeder vessels (Figure 2F). Type 1 CNV membranes are often mature, poorly respond to anti-VEGF therapy and have a worse visual prognosis compared to type 2 CNV.24-26
Type 2 (classic). This type of CNV is characterized by proliferation of neovascular tissue above the RPE, in the subretinal space. Type 2 membranes are consistent with classic leakage patterns of well-demarcated hyperfluorescence on conventional IVFA.4 Using structural OCT, type 2 neovascularization is often characterized by a subretinal hypereflective mass resting on top of the RPE and frequently has overlying intraretinal fluid (Figure 6). These membranes are usually well-defined complexes with OCT-A.
Mixed Type 1 and 2. CNV membranes may be partially located beneath the RPE and in the subretinal space. Mixed types are sometimes subclassified as predominantly classic (Figure 7). They can also be minimally classic when the subretinal component is either greater or less than 50% of the total lesion area.4,10
Figure 7 depicts the right eye of a 73-year-old who was being monitored closely for intermediate dry AMD in both eyes prior to the COVID-19 pandemic. During the pandemic, she cancelled twp appointments. Fifteen months after her last exam, she presented as a walk-in complaining of decreased vision in her right eye for at least six months. Her Snellen distance acuity in the right eye had declined from 20/20 to 20/100.
 |
|
Fig. 6. Type 2 neovascular exudative AMD. Color photo shows central gray-green subretinal thickening with surrounding subretinal hemorrhage. OCT reveals hyperreflective subretinal mass corresponding to a type 2 neovascular membrane with overlying intraretinal cystic edema. Click image to enlarge. |
 |
|
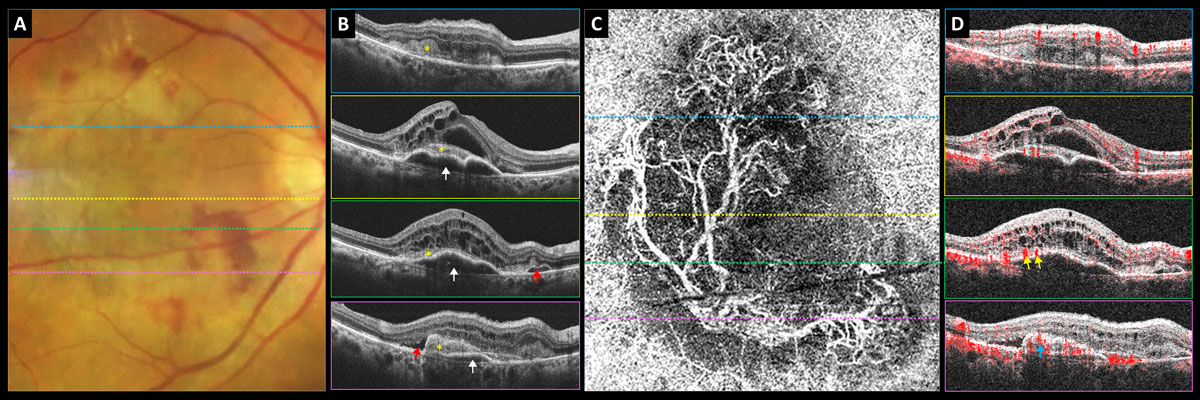
Fig. 7. Predominately type 2 neovascular exudative AMD: (A) color photograph, (B) structural OCT, (C) 6mm OCT-A outer retina choriocapillaris en-face display and (D) OCT-A B-scan overlay. White arrows point to PED, yellow asterisk shows subretinal neovascular membrane, red arrows point to subretinal fluid, yellow arrows point to subretinal feeder vessels and blue arrow points to subretinal neovascular membrane. Click image to enlarge. |
Fundus examination revealed central gray-green subretinal thickening with surrounding subretinal hemorrhage and minor exudate superiorly (Figure 7A). Her structural OCTs showed a mostly serous multi-lobed PED (white arrows) that became shallower inferiorly with overlying hyperreflective subretinal mass (subretinal neovascular membrane, yellow asterisk) and significant intraretinal cystic edema (Figure 7B). There was also some subretinal fluid (red arrows).
The 6mm OCT-A outer retina choriocapillaris enface display revealed a mature neovascular complex with large feeder vessels (Figure 7C). OCT-A B-scan overlay shows that the large feeder vessels are in the subretinal space above the PED (Figure 7D). Inferiorly, the subretinal mass is red and highly vascularized, suggestive of a subretinal neovascular membrane (Figure 7D).
Type 3 (retinal angiomatous proliferation). This is characterized by deep intraretinal neovascularization that often anastomoses with a sub-RPE neovascular membrane. Other structural OCT features suggestive of neovascular and/or exudative AMD include:
Fluid. Fluid at any level on OCT, including intraretinal (Figure 6), subretinal (Figure 2E and 3), and subRPE (serous PED, Figure 4) is suggestive of active exudative AMD. With OCT, fluid is hyporeflective or dark, and this imaging is superior to ophthalmoscopy in the detection of fluid.
Hemorrhage. A hemorrhage must be quite large to be visualized with OCT. Because blood is pigmented, it is hyperreflective and may block light transmission posterior to it (produce a posterior shadowing artifact). Ophthalmoscopy and color photography are superior to OCT in detection.
Exudate. Exudate may be intraretinal or subretinal. The exudative material itself is hyperreflective and is often associated with posterior shadowing. Anterior RPE pigment migration, a high-risk nonexudative AMD phenotype, may appear similarly on OCT imaging; thus, comparing OCT and photography is sometimes needed to determine whether intraretinal hyperreflective foci represent exudation or pigment migration.
Pigment epithelial detachment (PED).As mentioned previously, there are various types of PED and differentiating them with multimodal imaging is critical to determine whether or not the clinician should suspect exudation/neovascularization and refer out.11 Enhanced-depth imaging or swept-source OCT can be of value in differentiating different PED types since they improve posterior image resolution.11
Drusenoid PEDs are associated with non-exudative AMD and appear as round elevations of the RPE filled with homogeneous and uniformly mildly hyperreflective drusenoid material (Figure 1B). Purely drusenoid PEDs should not be associated with intraretinal or subretinal fluid.
In contrast, fibrovascular PEDs that contain type 1 neovascularization exhibit variable (non-homogenous or non-uniform) internal reflectivity, which may include horizontally oriented multilaminate hyper-reflective sheets due to the lateral proliferation of new blood vessels (Figure 3).10,11 The elevation of the RPE is also often irregular in contour or the PED is multilobulated.10
Hemorrhagic and fibrovascular PEDs are always associated with exudative and/or neovascular AMD.
Serous PEDs exhibit a smooth, dome-shaped elevation of the RPE and are internally dark or optically empty since they are filled with clear fluid (Figure 4).10 Bruch’s membrane is often visible at the base of the PED as a thin hyperreflective line.10 While serous PEDs may be a manifestation of dry AMD, they are more often a manifestation of wet AMD and should be evaluated with fluoresein angiography and indocyanine green angiography to look for a treatable neovascular source.11,21
OCT-A. This instrument allows the clinician to directly visualize the choroidal neovascular membranes themselves, not just the secondary effects such as fluid identified with structural OCT.27,28 Performing OCT-A provides high-resolution, detailed images of CNV complexes, allowing the clinician to assess their shapes and morphologic patterns.1,28 In fact, the sensitivity and specificity for CNV detection using a combination of cross-sectional and en-face OCT-A imaging analysis approaches that of IVFA.4,23 OCT-A is also the only modality that can directly image high-risk nonexudative neovascular membrane. Therefore, OCT-A should be performed periodically in eyes clinically graded as having intermediate stage non-exudative AMD to look for early neovascularization.
The outer retina or outer retina choriocapillaris preset en-face display was specifically designed to aid in detection of CNV (Figures 2C, 2F, 5C and 7C). The segmentation boundaries vary depending upon the OCT brand, but typically include some combination of photoreceptors, RPE and choriocapillaris (Figure 5D). OCT-A can also be of great value in differentiating vascularized (Figure 2) from non-vascularized PEDs (Figure 1).11
Fluorescein angiography and indocyanine green angiography. Although invasive, fluoresein angiography can add value in detecting and classifying neovascular membranes as well as determining the degree of exudative activity.4 Indocyanine green angiography is of particular use in imaging the choroidal vasculature and is important in the diagnosis of polypoidal choroidal vasculopathy, which is considered a subtype of AMD.4
Step 5: Refer for Treatment
Intravitreal anti-VEGF is the standard first-line therapy for treating wet AMD, and early detection and prompt treatment results in better visual outcomes.4 Immediately refer to a retina specialist, if available, or an ophthalmologist experienced in retinal disease whenever a diagnosis of new onset exudative AMD is made or highly suspected.3,4 The consultative exam should be scheduled within a few days and no longer than one week from the diagnosis.
 |
Step 6: Prepare for the Long Haul
Patients who have undergone anti-VEGF therapy and are either no longer receiving injections or receiving injections on an as-needed or treat-and-extend protocol may still suffer a relapse that requires prompt treatment or an injection sooner than was originally planned. Patients with worsening symptoms should be promptly examined and if new or worsening exudative fundus or imaging features such as hemorrhage or fluid are found, the managing ophthalmologist or retinal specialist should be informed.
Prompt treatment of wet AMD is associated with better long-term visual outcomes, and many cases of severe loss can actually be prevented through early detection and referral.3,4 Recognizing fundus findings suggestive of exudation, such as hemorrhage and exudate, and using imaging technologies, especially OCT, will allow earlier detection of exudation in hopes that your patients’ vision can be saved.
Dr. Majcher is is an associate professor and the director of residency programs at the Oklahoma College of Optometry Northeastern State University. She is a paid speaker and consultant for Regeneron Pharmaceuticals and Carl Zeiss Meditec. She is also a paid advisory board member for Apellis and has received non-financial support from Roche.
Dr. Petry is a primary care resident at the Oklahoma College of Optometry Northeastern State University. She has no financial disclosures.
Dr. Das is a primary care resident at the Oklahoma College of Optometry Northeastern State University and currently pursuing her Fellowship of the American Academy of Optometry. She has no financial disclosures.
1. Gheorghe A, Mahdi L, Musat O. Age-related macular degeneration. Rom J Ophthalmol. 2015;59(2):74-7. 2. Centers for Disease Control and Prevention. Vision Health Initiative. Common Eye Disorders and Diseases. www.cdc.gov/visionhealth/basics/ced/index.html#:~:text=external%20icon-,Cataract,loss%20in%20the%20United%20States. June 3, 2020. Accessed April 17, 2022. 3. Optometric Association Consensus Panel. Care of the patient with age-related macular degeneration optometric clinical practice guideline 1994, revised 1999 and reviewed 2004. www.aoa.org/practice/clinical-guidelines/clinical-practice-guidelines?sso=y. Accessed April 17, 2022. 4. American Academy of Ophthalmology Retina/Vitreous Preferred Practice Pattern Panel. Age-related macular degeneration preferred practice pattern. www.aao.org/preferred-practice-pattern/age-related-macular-degeneration-ppp. July 2019. Accessed April 17, 2022. 5. Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477-85. 6. Ferris FL, III, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102(11):1640-2. 7. Arroyo JG, Gardiner MF, Schmader KE, et al. Age-related macular degeneration: clinical presentation, etiology, and diagnosis. UpToDate. February 2022. 8. Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology. 2001;108(4):697-704. 9. Rein DB, Wittenborn JS, Zhang X, et al. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Arch Ophthalmol. 2009;127(4):533-40. 10. Salmon, JF. Kanski’s Clinical Ophthalmology: A Systematic Approach. Ninth ed., Elsevier. 2020. 11. Karampelas M, Malamos P, Petrou P, et al. Retinal pigment epithelial detachment in age-related macular degeneration. Ophthalmol Ther. 2020;9(4):739-56. 12. Bacci T, Essilfie JO, Leong BCS, Freund KB. Exudative non-neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2021;259(5):1123-34. 13. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene and zinc for age-related macular degeneration and vision loss: AREDS report number 8. Arch Ophthalmol. 2001;119(10):1417-36. 14. Risk factors for choroidal neovascularization in the second eye of patients with juxtafoveal or subfoveal choroidal neovascularization secondary to age-related macular degeneration. Macular Photocoagulation Study Group. Arch Ophthalmol. 1997;115(6):741-7. 15. Lee J, Choi S, Lee CS, et al. Neovascularization in fellow eye of unilateral neovascular age-related macular degeneration according to different drusen types. Am J Ophthalmol. 2019;208:103-10. 16. Raman R, Biswas S, Gupta A, et al. Association of macular pigment optical density with risk factors for wet age-related macular degeneration in the Indian population. Eye (Lond). 2012;26(7):950-7. 17. Flamendorf J, Agrón E, Wong WT, et al. Impairments in dark adaptation are associated with age-related macular degeneration severity and reticular pseudodrusen. Ophthalmology. 2015;122(10):2053-62. 18. Bailey ST, Gao SS, Flaxel CJ, et al. Early detection of choroidal neovascularization with OCT angiography. ARVO Annual Meeting Abstract. June 2017. 19. Bailey ST, Thaware O, Wang J, et al. Detection of nonexudative choroidal neovascularization and progression to exudative choroidal neovascularization using OCT angiography. Ophthalmol Retina. 2019;3(8):629-36. 20. de Oliveira Dias JR, Zhang Q, Garcia JMB, et al. Natural history of subclinical neovascularization in nonexudative age-related macular degeneration using swept-source OCT angiography. Ophthalmology. 2018;125(2):255-66. 21. Introini U, Casalino G. Serous PED. www.amdbook.org/content/serous-ped-0. July 2017. 22. van Grinsven MJ, Theelen T, Witkamp L, et al. Automatic differentiation of color fundus images containing drusen or exudates using a contextual spatial pyramid approach. Biomed Opt Express. 2016;7(3):709-25. 23. Faridi A, Jia Y, Gao SS, et al. Sensitivity and specificity of OCT angiography to detect choroidal neovascularization. Ophthalmol Retina. 2017;1(4):294-303. 24. Kim JH, Kim JY, Lee DW, et al. Fibrovascular pigment epithelial detachment in eyes with subretinal hemorrhage secondary to neovascular AMD or PCV: a morphologic predictor associated with poor treatment outcomes. Sci Rep. 2020;10(1):14943. 25. Suzuki M, Nagai N, Izumi-Nagai K, et al. Predictive factors for non-response to intravitreal ranibizumab treatment in age-related macular degeneration. Br J Ophthalmol. 2014;98(9):1186-91. 26. Hoerster R, Muether PS, Sitnilska V, et al. Fibrovascular pigment epithelial detachment is a risk factor for long-term visual decay in neovascular age-related macular degeneration. Retina. 2014;34:1767-73. 27. De Carlo TE, Romano A, Waheed NK, Duker JS. A review of optical coherence tomography angiography (OCTA). Int J Retina Vitreous. 2015;1(1):1-15. 28. Jia Y, Bailey ST, Wilson DJ, et al. Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology. 2014;121(7):1435-44. |

