Earn New Surgical SkillsOptometric surgery is increasingly becoming a core responsibility of the profession. Today, 17 states (and counting) allow ODs to perform laser or other minor surgical procedures. Here to offer tips and advice on surgery implementation and technique is the December 2023 issue of Review of Optometry, the magazine's 30th annual surgery report. Inside, experts offer advice on performing SLT, equipping your office for surgery and handling complications of in-office procedures, along with a review of the newest IOLs on the market. Check out the other articles featured in the December issue: |
As cataract surgery maintains its title as one of the most prevalent surgeries in the United States, technological advancements have resulted in the availability of over 100 intraocular lenses (IOLs) on the market.1 As optometrists, it is crucial for us to be well-informed about the expected postoperative visual outcomes and potential side effects patients may experience following cataract surgery so that we can provide effective care and guidance during preoperative screening and postoperative follow-up visits. Here, we will delve into the new and improved lens types making waves in the US today.
Innovative IOLs
The rise of this new technology boom can be attributed to the widespread use of smartphones, tablets and computers in our daily lives. There is now a growing demand on intermediate vision, and some of these new IOLs aim to offer patients the opportunity for not only improved vision but also reduced dependence on glasses or contact lenses at intermediate and near tasks.
Historically, there have been two types of lenses used at the time of cataract surgery—monofocal/toric monofocal lenses and multifocal IOLs. Monofocals typically aim to provide one focal point of clear vision, such as distance vision when aiming for emmetropia. However, monovision can also be used to create two main areas of clear vision, typically done in patients with a history of monovision, but these patients uncorrected can have a lack of intermediate vision. Multifocal IOLs, of course, use multiple foci. Despite the use of this term multi- since the early 1990s, many doctors have historically considered these lenses to be more of a bifocal-like correction. Additionally, these initial lenses created glare and halos that were very bothersome to patients.
To achieve a broader range of vision while minimizing side effects, recent developments in lens technology have led to two main categories. One prioritizes distance and intermediate vision while the other encompasses distance, intermediate and near vision, aiming to provide a greater range of functional use. The distance and intermediate vision groups of lenses are called monofocal plus lenses or extended-depth-of-focus (EDOF) lenses, and the distance, intermediate and near vision lenses are called trifocal lenses or trifocal-like multifocal lenses.
 |
|
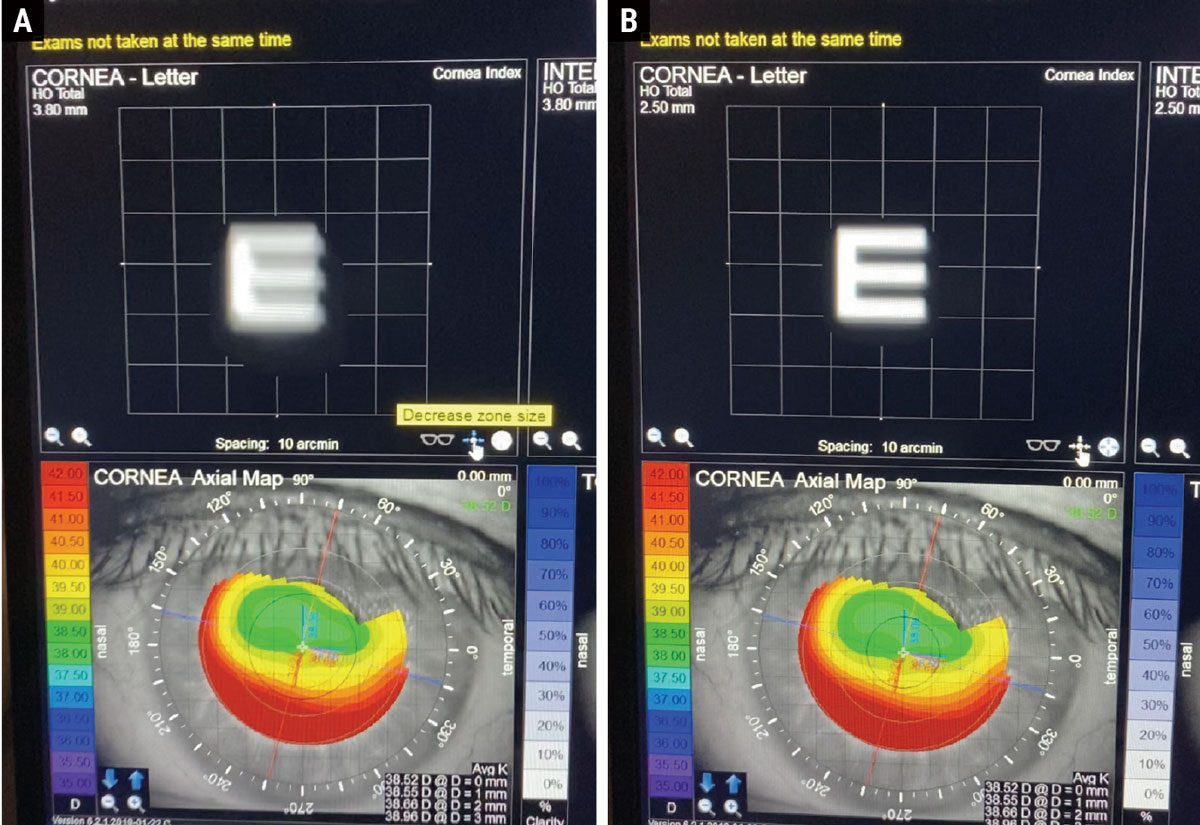
A post-refractive myopic LASIK patient presenting for a cataract eval with a decentered ablation. Here, the iTrace is able to show the benefit of a small-aperture IOL. Left is the simulated image of an E with the patient’s normal pupil size (A). Right shows the improvement in the simulated image with a small aperture IOL (B). Click image to enlarge. |
Distance, Intermediate and Near Vision Lenses
Trifocal and trifocal-like multifocal IOLs have gained substantial popularity in recent years due to their ability to provide correction of distance, intermediate and near vision without much need for additional glasses. A recent meta-analysis showed a spectacle independence rate of 91.6% of patients receiving trifocal IOLs in both eyes.2
Despite the extended range of vision that can be achieved, post-op expectations, including side effects, should be discussed with every patient in order to select a lens that best suits the patient’s goals. Trifocal IOLs are not perfect and setting and maintaining expectations before surgery is imperative. One of the best ways to determine these could be a patient questionnaire. In a recent study, personality traits such as low conscientiousness, extroversion and high neuroticism significantly influenced the happiness and quality of vision perception at six months after bilateral multifocal lens implantation.3 In addition to assessing the patient’s personality, their ocular surface and overall eye health must be in optimal condition for them to get a trifocal lens.
Next, let’s look at several recent products in this category:
Clareon PanOptix Trifocal IOL (Alcon). This is currently the only lens in the US labeled as a trifocal. It is known for its ability to provide vision at multiple distances, including near, intermediate and far. Despite this label, it functions as a quadrifocal, with two of its foci focused at distance.4 One main advantage of PanOptix includes the excellent range of vision, with an average visual acuity of 20/25 or better from distance to 40cm.5 Another advantage of the PanOptix lens and its non-sequential diffractive optics is its capability to achieve clear vision at intermediate distances (around 24 inches) and near distances (around 16 inches), which are commonly used for tasks involving close-up work and reading.
The PanOptix IOL has the ability to correct up to 2.60D of astigmatism. It is a diffractive IOL, so the need for good overhead lighting should be discussed so the patient is aware near vision is dependent on the amount of light directed at the near target. In a study of 65 subjects who underwent bilateral PanOptix implantation, all patients were spectacle independent for distance vision and only two needed over-the-counter readers or near correction.6
The greatest limitation to this lens, as in any multifocal, is its optical design, which uses rings for diffraction. These rings will be seen at night by patients and have to be discussed prior to surgery. In most cases, the halos or glare caused by the rings is neuro-adapted by patients over a three to six-month period.7 The side effect profile of the PanOptix is much better than the initial multifocal lenses of three decades ago, and expectations among practitioners and patients alike need to reflect current performance. A PanOptix IOL OU study of 55 patients showed that at six months there was a 98.2% patient satisfaction rate and only one patient had nocturnal glare that affected their life.5 The good news is the happiness usually stays. The largest, longest term study so far, of over 1,000 eyes, showed three years of stable visual acuity.8
Tecnis Synergy OptiBlue IOL (Johnson & Johnson Vision). This lens has a diffractive surface derived from a combination of EDOF (distance & intermediate) and multifocal (distance and near) concepts. It is designed to correct chromatic aberration, spherical aberration and provide a range of vision from distance to near. The Synergy lens is referred to as a continuous range of vision lens by the manufacturer; however, when looking at the optical qualities of the lens using the modulation-transfer-function principle, simulated defocus curves and defocus curves, it behaves similar to a trifocal lens.9-11 Several studies refer to the Synergy as a trifocal lens, and we believe this term simplifies what this lens is capable of doing to patients, optometry students, optometry residents and doctors.
This trifocal-like lens offers vision at distance, intermediate and near, but its near add power is stronger than what’s available in the PanOptix IOL. This allows patients to potentially have better near vision for closer working distances.11 This can be beneficial for patients with shorter working lengths or for myopes who remove their glasses for near work. Similar to other multifocals, it does require a relatively healthy eye and the need for increased lighting for the best possible vision.
PanOptix vs. Synergy. Different surgical groups have their own preferences on which lens is their primary presbyopia-correcting IOL. There are several studies that have put these two lenses head-to-head to see if there is a clear winner. The first is a three-month visual outcomes comparison, which showed that when patients were corrected for distance, the PanOptix and Synergy distance vision was comparable. However, when near vision was evaluated, there was a slight statistical edge for Synergy over PanOptix.10 A separate study looked at uncorrected distance, intermediate and near vision between Synergy and PanOptix. PanOptix had better distance vision, while the average uncorrected near visual acuity was superior in the Synergy group. There was no difference in the intermediate vision. When looking at the halo and glare complaints, more patients complained of them at one and three months with the Synergy group, but by six months, glare and halos were the same in both groups. A stat we often tell patients: Only 13% in the study reported halos in both groups at six months.12 Although not statically significant, the PanOptix patients demonstrated better uncorrected distance visual acuity sooner than the Synergy group.
So, which lens to choose? There is no right answer, as both lenses have advantages and overlap in most categories. Essentially, it comes down to communication with the patient and which area of their vision they prioritize as significant. If that patient prioritizes near vision, Synergy may be the better selection. If they prioritize distance vision, PanOptix may be the better selection.
One interesting fact—when looking at the latest Market Scope data, many patients are current selecting PanOptix over Synergy.
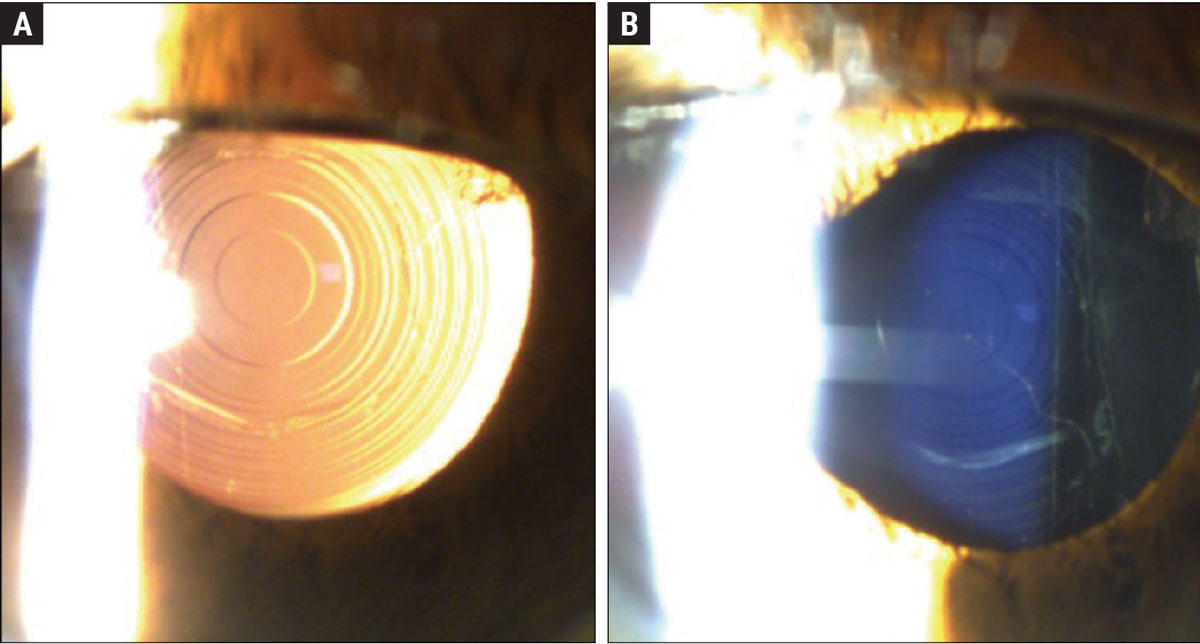 |
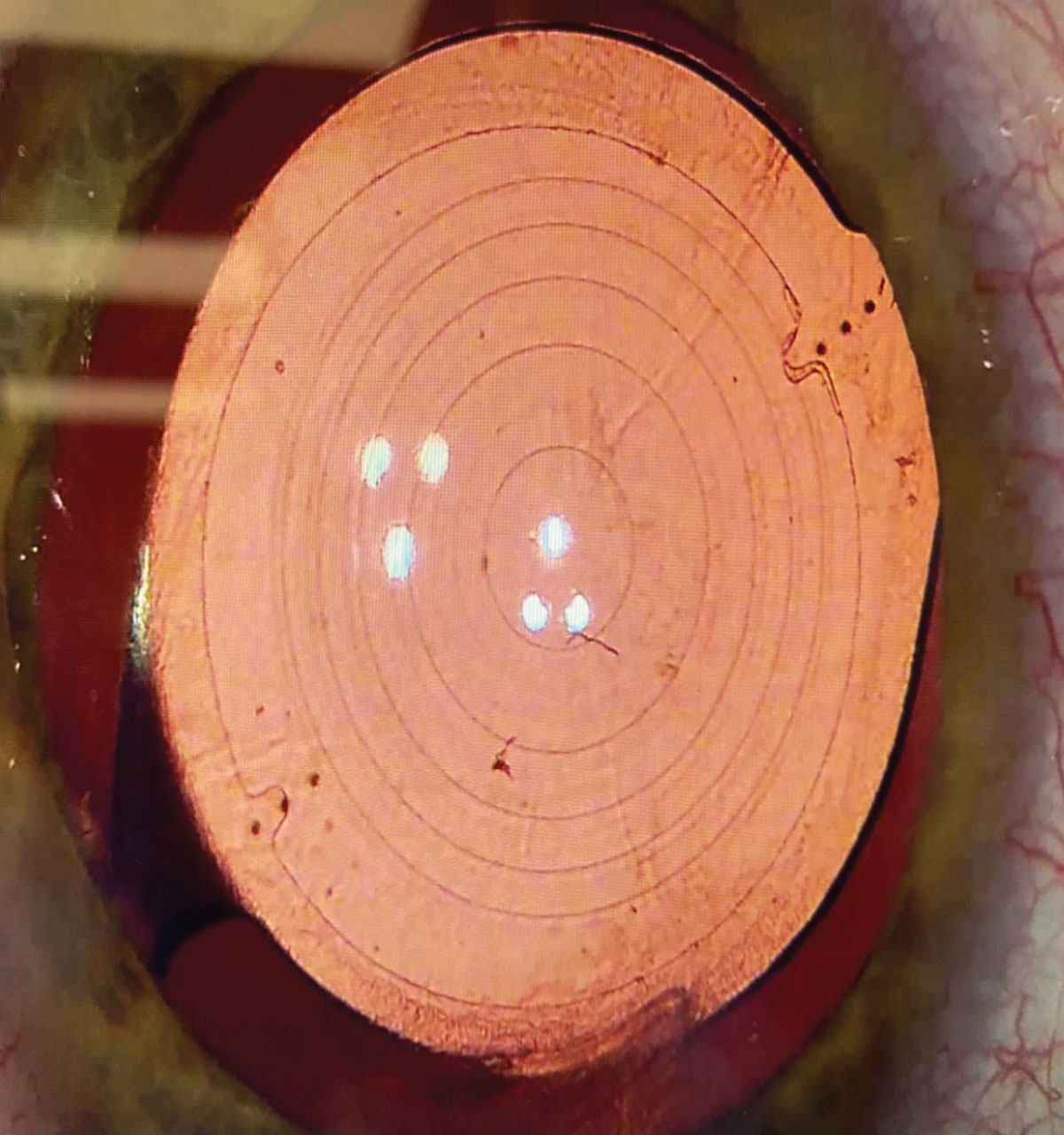
This patient has a Symfony OptiBlue IOL. Figure (A) shows the echellette diffractive design with retroillumination. When viewing the lens directly, you can appreciate the blue hue of the lens (Figure B). The OptiBlue filter blocks the shortest wavelengths of light that produce the most light scatter, helping to mitigate halo, glare and starbursts when driving at night. Click image to enlarge. |
New Kids on the Block
Some really interesting new optical concepts have been introduced in recent years. Here are a few notable ones.
ClearView3 Mutifocal (Lenstec). Previously known as SBL-3, this is a segmented bifocal IOL. Unlike the trifocal IOLs, this lens doesn’t use diffractive optics and doesn’t have an intermediate focus. Instead, it uses two segmented optical zones, similar to a traditional lined bifocal or a translating bifocal rigid gas permeable contact lens. The top zone is designed for pure distance and the bottom for near. The Clearview 3 has shown to reduce halos, although patients have reported winged dysphotopsias. Limitations include the lack of lens toricity and intermediate vision. Consider this lens in a patient who wants monofocal-like optics at distance with some near vision.
EDOFs. Even though trifocals have significantly improved patient satisfaction and range of vision, there are patients who want to minimize the risk of having halos at night and want more vision than just a monofocal. Thus, EDOFs have come onto the market. Rather than creating different foci, EDOF lenses elongate a single focal point, providing distance and some intermediate vision. These lenses have been gaining traction not only in patients with optimal eye health, but those with a history of refractive surgery and mild pre-existing ocular conditions such as mild epiretinal membranes, primary open-angle glaucoma or age-related macular degeneration.
Tecnis Symfony IOL (Johnson & Johnson Vision). This the first IOL to have FDA approval as an EDOF lens. Some think of Symfony more as a low-add multifocal lens due to it being a diffractive lens. Similar to the Vivity lens (discussed below), Symfony provides around 1.53D of add and corrects astigmatism from 1D to 2.6D.1 The Symfony is currently on its second version—Symfony OptiBlue—which has the same defocus curve as the first version but has shown in optical bench studies to mitigate dysphotopsias and improve contrast.13
Vivity (Alcon). This EDOF IOL aims to stretch the wavefront to provide a range from distance to intermediate vision. Vivity is able to achieve approximately a +1.5D of add power and can correct corneal astigmatism up to 2.45D.1 The Vivity IOL was the first EDOF in which we were able to see improved intermediate vision compared to a monofocal IOL, while having less halo symptoms at night compared to a trifocal IOL.14 For example, a study showed at one month—a point where neuroadaptation was still underway—Vivity had 85% of patients report little to no glare or halos compared with 69% of patients who had PanOptix. In a double-blinded prospective study comparing Symfony to Vivity, 60% of Vivity patients reported no glare compared to 88% of Vivity patients reporting no halo.
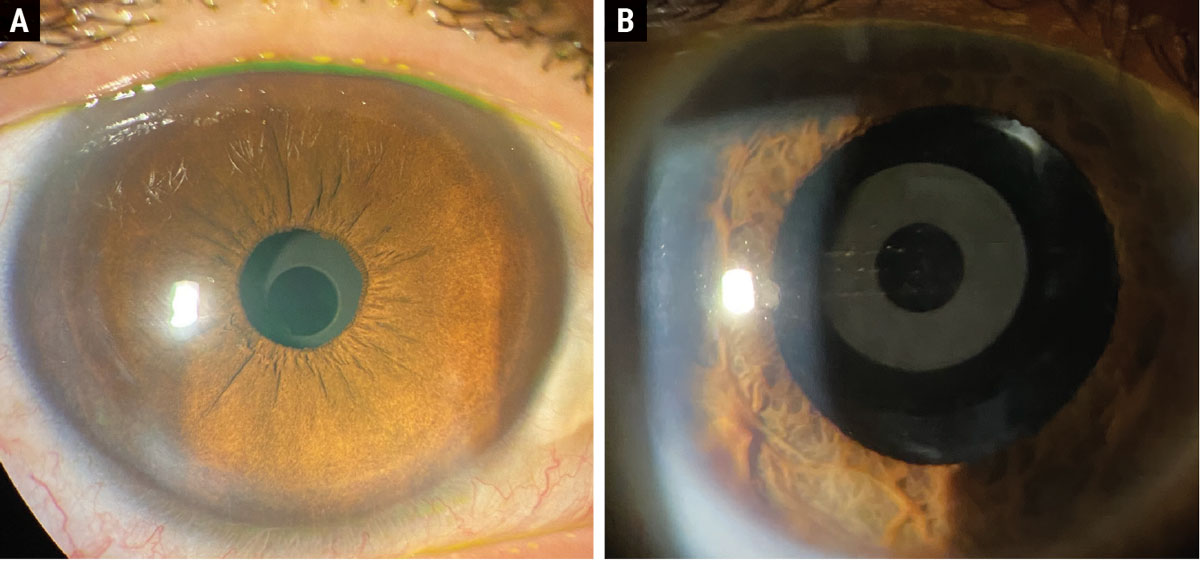 |
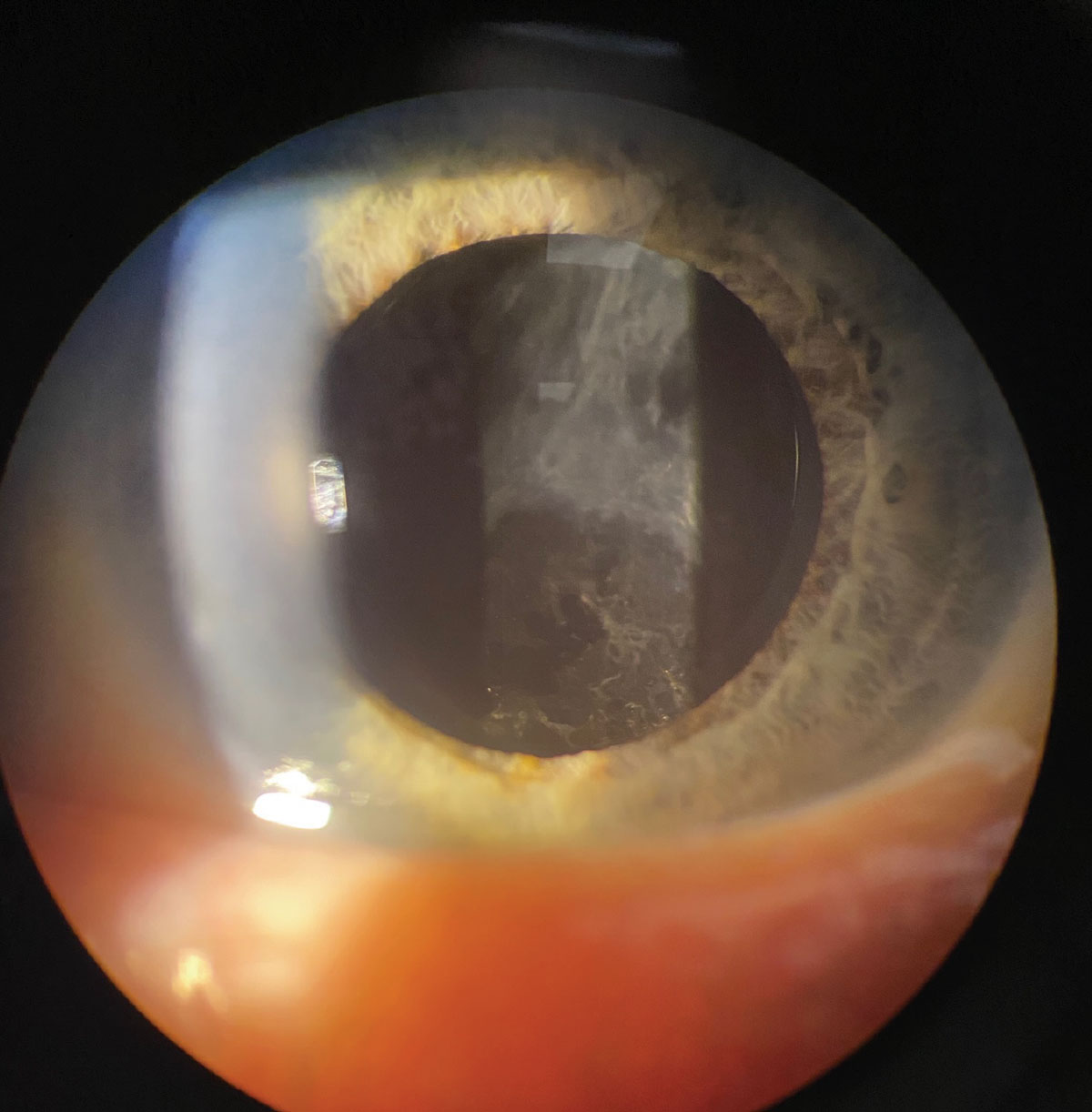
The Apthera pinhole IOL in a post-refractive patient undilated (A) and dilated (B). Click image to enlarge. |
Apthera IOL (AcuFocus). Previously known as IC-8, this is a new, small-aperture IOL that uses pinhole optics to provide an EDOF effect. Despite the lens having a small aperture (3.23mm circular mask with 1.36mm aperture), the mask has 3,200 microperforations to aid in minimizing any negative effect on field of vision.11
In clinical trials, this lens was implanted in the non-dominant eye (about -0.75D target), while the dominant eye had a traditional monofocal lens implanted.11 At six months, the binocular average uncorrected distance visual acuity was 20/20, uncorrected intermediate visual acuity was 20/22 and uncorrected near was 20/31.11 As expected, out of all the lenses Apthera has the lowest risk of glare and halos. The best patient for this might be one who has an atypical cornea, such as a decentered LASIK ablation. The smaller aperture will decrease the overall aberrations and potentially provide better vision than they had previously achieved.
This lens has been used outside of the US in patients who would benefit from a smaller pupil size to reduce the effect of corneal aberrations. Notably, patients with a history of radial keratotomy, decentered photorefractive keratectomy or LASIK, aniridia or even keratoconus may potentially benefit from this lens.16 Limitations include dimming of the vision on the side where the Apthera is implanted and the absence from the market of a toric version; however, the pinhole effect can “mask” around 1.5D of astigmatism.17
We tell EDOF patients they will still need reading glasses; we don’t want to overpromise. There are plenty of surgical groups who will offset these EDOF IOLs; for example, plano target in the dominant eye and -0.75D sph target in the nondominant eye, to reduce the need for reading glasses.
Overall, the biggest benefit of EDOFs compared with traditional multifocals is the reduction in glare symptoms. Thus, if we have a patient who wants to minimize the chance of halos postoperatively, we lean towards an EDOF or an enhanced monofocal lens (more on this shortly).10
 |
| Click image to enlarge. |
Monofocal Plus IOLs
In order for a lens to earn the title of an EDOF, it must meet four of the American National Standard Institute (ANSI) criteria (Table 1). There are lenses such as the Tecnis Eyhance IOL (Johnson & Johnson Vision), whose EDOF that provides an add of 1.3D does not currently not meet the ANSI criteria.
The question then becomes, what do we call lenses that create some EDOF effect but are not enough to warrant the term EDOF by ANSI standards? Terms in the literature for these lenses include monofocal-plus, enhanced monofocal or EDOF monofocal. Other lenses that fit in this category are the Light Adjustable Lens (RxSight), RayOne EMV (Rayner), enVista IOL monofocal (Bausch + Lomb) and enVista Aspire IOL (Bausch + Lomb). We like to refer to these as monofocal-plus lenses to our patients.
Monofocal-plus IOLs lenses provide comparable distance visual acuity to a basic monofocal lens and improved intermediate visual acuity compared to a basic monofocal lens. The question is, do patients notice the difference in EDOF abilities between the monofocal plus lenses and the EDOF lenses? Three studies showed no significant differences for intermediate visual acuity between Eyhance and Vivity.20-22 However, Eyhance was inferior to the EDOF lenses at near.
 |
|
The Ally femtosecond laser (LensAR) creates markings in the lens capsule to precisely place toric IOLs. Here, a toric trifocal lens was used. If a lens is rotated postoperatively, which usually is suspected with a residual mixed refraction, dilating the patient can help to evaluate the alignment of the toric IOL. Click image to enlarge. |
Tecnis Eyhance IOL. This is one of the most studied monofocal-plus lenses. Its unique design uses a modified anterior aspheric surface that leads to a progressive defocus of up to 1D. The advantage of Eyhance is its excellent distance vision with relatively good intermediate vision and a reduced risk of visual disturbances. Limitations for this lens, similar to the EDOF lenses, is the requirement for reading glasses for most near activities after surgery.
One study comparing Eyhance to Vivity looked at 76 eyes of 38 patients.20 Uncorrected distance vision and uncorrected intermediate vision were comparable in both groups. The two groups had no difference between halo and glare perception. However, Vivity provided better uncorrected near vision and higher spectacle independence for near vision. No difference in patient satisfaction was noted when comparing the lenses.
Light-Adjustable Lens (LAL). This IOL was approved by the FDA in 2017, and many doctors around the country have expressed their excitement for the lenses since then due to the ability to alter lens power after implantation.19 The LAL offers the advantage of post-implantation adjustability, allowing for modifications of the patient’s refraction using the RxSight Light Delivery Device (LDD), wherein macromers within the lens can be precisely tuned using UV light delivered by the LDD device to achieve the targeted refraction.
For example, let’s say you have a monovision patient who wants monovision after cataract surgery. One month after surgery, their distance eye is great with no complaints, but the patient desires more near vision. The LAL has some EDOF technology in it, so we refer to this as a monofocal-plus lens, and you can actually induce more EDOF in the lens in the post-op adjustment by doing a specific light treatment.23 Once the patient has a stable refraction, you can use the LDD to adjust the refraction from -1.25D sph to -1.75D sph to achieve the near vision the patient desires. After the patient is at their desired refractive status, the lens performance is locked in with the LDD.
The lock-in step of the Light-Adjustable Lens process uses the LDD to exhaust all macromers, thereby stabilizing the patient’s refraction status and preventing subsequent changes. Before the lock-in step, the lens can be adjusted up to three times. In any given adjustment, the lens can be modified up to 2D of hyperopia, myopia or astigmatism.
Another example where the LAL could be used is a radial keratotomy patient or any post-refractive surgery patient.24 These previous refractive procedures can make the postoperative refractive status less predictable. If a radial keratotomy patient ends up +2D sph or +3D sph one month postoperatively, if the LAL was used we will have a chance to achieve plano postoperatively through a series of LDD treatments without having to exchange the IOL.
This lens does have its own set of limitations, as it requires patients to wear specific UV-blocking glasses provided by RxSight in the post-op period. The lens must be worn while outdoors for the full length of postoperative care until the IOL is locked into place and can no longer be altered by UV light. The amount of time before the lens is locked in varies from patient to patient depending how many treatments are needed after surgery. This process can take anywhere from approximately one month to several months.
Many states and surgical practices have optometrists doing the post-op measurements and adjustments. One open access center has two optometrists providing LAL postoperative care for 16 surgeons.25 We only expect the role of the optometrist to increase in the LAL postoperative period.
As this article was under review, a new lens came out—the enVista Aspire (Bausch + Lomb), which “uses an optical modification of the posterior aspheric surface to create a small continuous increase in IOL power within the central 1.5mm diameter to slightly extend the depth of focus,” according to company literature.
We believe we will continue to see lenses that use spherical aberrations or lenses that slightly modify the central zone to provide more monofocal-plus or EDOF lens options.
 |
Posterior capsular opacification can be seen in this image. In premium lenses, especially those correcting more than one vision zone such as an EDOF or trifocal lens, mild posterior capsular opacification can cause severe halo/glare effects. Click image to enlarge. |
Multifocals vs. EDOFs vs. Monofocal Plus
Oftentimes when we are discussing lenses with patients, we come to the question, “Which lens is best for the patient—trifocal, EDOF or monofocal-plus?” We should first ask what the patient is looking to achieve after surgery, then inquire about their personality and tolerance for possible halos after six months.
It’s good to keep a few facts and additional studies in mind when making this decision. First, a 2022 study looked at PanOptix and compared it to EDOF lenses such Vivity, Symfony and Eyhance. There was no statistical difference between uncorrected binocular distance vision between any of the listed lenses.26 Overall, these lenses have about the same distance vision if they are “on target,” meaning minimal to no residual refractive error, which means in a healthy eye, the amount of near vision demand, cost and tolerance to halos drive the lens type we choose. Secondly, some studies have shown EDOF lenses produce less dysphotopsias.14 In one study, 85% of Vivity patients stated they had no or very little glare and halos compared to 69% of patients with PanOptix.14
Giving patients the ability to see at a distance and to be able to see the floor while walking or their computer with these EDOF or monofocal-plus lenses, with little to no side effects, is one of the biggest reasons these lenses have been taking the US and Europe by storm. If we have better options than just our traditional monofocal lenses, why not use them? In addition, many surgical groups have been doing blended vision or mini-monovision to provide these patients with more near vision. Typically, the dominant eye is targeted for plano and the non-dominant eye is targeted around -0.75D sph.
Interestingly, multiple recent meta-analyses have showed no difference in patient satisfaction or between EDOF and trifocal IOLs.26-28 Ultimately, some doctors shy away from trifocals because of fear of halos, but these studies confirm what we see in clinical practice—patients do well with trifocal lenses. They also do well with EDOF lenses. As indicated by the study findings, patient contentment can be attained with any of these lens technologies, if expectations are effectively established and communicated.
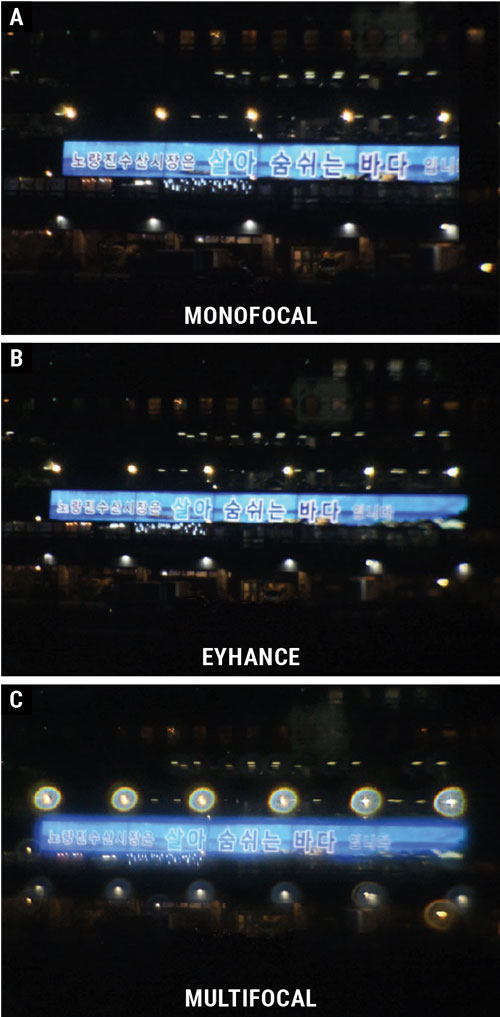 |
A recent study simulated images for patients when looking at a distance target at night. Oftentimes, visuals such as this can be helpful when describing to a patient what to expect postoperatively. Click image to enlarge. |
Takeaways
Optometrists will continue to play a vital role in discussing the different types of IOL choices and setting expectations. By understanding the design philosophies and the clinical performance of newer IOLs, we can help guide patients toward the best visual outcome for each patient’s specific vision needs. With the advancements in scientific research, enhancements in intermediate vision have been achieved through the use of trifocal IOLs, trifocal-like IOLs, EDOF IOLs and monofocal-plus IOLs, offering the capacity to restore visual acuity in numerous patients and elicit a high level of satisfaction.
Dos and Don'ts of Cataract ComanagementBy Katie Gilbert Spear, OD, JD, Pensacola, FL Comanagement has been, and continues to be, an integral part of many ODs’ practices. It has become even more important as medical offices get busier and the ability to get patients into specialists like ophthalmologists in a timely manner becomes more challenging. A recent study projects that by 2035, the number of full-time ophthalmologists will decrease by 2,650, or 12%. However, 5,150 full-time ophthalmologists will be needed by 2035—an increase of 24%. This discrepancy results in a supply and demand mismatch of about 30%.1 This shows that comanagement is needed now and will be needed even more in the future.
|
Dr. Saenz is co-founder of LASIK San Antonio and an assistant professor at the University of Incarnate Word Rosenberg School of Optometry. Dr. Lopez is the Director of Optometric Services at Castle Hills Eye Specialists. Dr. Sepulveda is the co-founder of LASIK San Antonio and founder of Castle Hills Eye Specialists. They have no financial disclosures.
1. Rampat R, Gatinel D. Multifocal and extended depth-of-focus intraocular lenses in 2020. Ophthalmology. 2021;128(11):e164-85. 2. Zhu D, Ren S, Mills K, et al. Rate of complete spectacle independence with a trifocal intraocular lens: a systematic literature review and meta-analysis. Ophthalmol Ther. 2023;12(2):1157-71. 3. Pinheiro RL, Raimundo M, Gil JQ, et al. The influence of personality on the quality of vision after multifocal intraocular lens implantation. Eur J Ophthalmol. 2023;11206721231176313. 4. Kohnen T, Lwowski C, Hinzelmann L, et al. Presbyopia correction in astigmatic eyes using a toric trifocal intraocular lens with quadrifocal technology. J Refract Surg. 2020;36(10):638-44. 5. Ma J, Sun X, Liu Y, Liu Y. Observation of visual quality after femtosecond laser-assisted cataract surgery combined with trifocal intraocular lens implantation. Comput Math Methods Med. 2022(2):1-8. 6. Galvis V, Escaf LC, Escaf LJ. Visual and satisfaction results with implantation of the trifocal PanOptix intraocular lens in cataract surgery. J Optom. 2022;15(3):219-7. 7. Rosa AM, Miranda ÂC, Patrício MM, et al. Functional magnetic resonance imaging to assess neuroadaptation to multifocal intraocular lenses. J Cataract Refract Surg. 2017;43(10):1287-96. 8. Jeon S, Moon K, Kwon H. Long-term clinical outcomes after trifocal intraocular lens implantation: a retrospective observational study. J Refract Surg. 2023;39(4):236-41. 9. Łabuz G, Yan W, Baur ID, et al. Comparison of five presbyopia-correcting intraocular lenses: optical-bench assessment with visual-quality simulation. J Clin Med. 2023;12(7):2523. 10. Dick HB, Ang RE, Corbett D, et al. Comparison of three-month visual outcomes of a new multifocal intraocular lens vs a trifocal intraocular lens. J Cataract Refract Surg. 2022;48(11):1270-6. 11. Yan W, Łabuz G, Khoramnia R, Auffarth GU. Trifocal intraocular lens selection: Predicting visual function from optical quality measurements. J Refract Surg. 2023;39(2):111-8. 12. Moshirfar M, Stapley SR, Corbin WM, et al. Comparative visual outcome analysis of a diffractive multifocal intraocular lens and a new diffractive multifocal lens with extended depth of focus. J Clin Med. 2022;11(24):7374. 13. van der Mooren M, Alarcon A, Jenkins Sanchez MD, Chang DH. Effect of violet light-filtering and manufacturing improvements in an extended depth-of-focus IOL on visual performance, Clin Ophthalmol. 2023;17:701-9. 14. Hovanesian JA, Jones M, Allen Q. The Vivity extended range of vision IOL vs the PanOptix trifocal, ReSTOR 2.5 active focus and ReSTOR 3.0 multifocal lenses: a comparison of patient satisfaction, visual disturbances and spectacle Independence. Clin Ophthalmol. 2022;16:145-52. 15. IC-8 Apthera Intraocular Lens - P210005. FDA.gov. July 22, 2022. Accessed September 2023. www.accessdata.fda.gov/cdrh_docs/pdf21/P210005B.pdf. 16. Sánchez-González JM, Sánchez-González MC, De-Hita-Cantalejo C, et al. Small aperture IC-8 extended depth-of-focus intraocular lens in cataract surgery: a systematic review. J Clin Med. 2022;11(16):4654. 17. Ang RE. Small-aperture intraocular lens tolerance to induced astigmatism. Clin Ophthalmol. 2018;12:1659-64. 18. Guarro M, Sararols L, Londoño GJ, et al. Visual disturbances produced after the implantation of three extended depth-of-focus intraocular lenses vs one monofocal intraocular lens. J Cataract Refract Surg. 2022;48(12):1354-9. 19. Fernández J, Rocja-de-Lossada C, Zamorano-Martin F, et al. Positioning of enhanced monofocal intraocular lenses between conventional monofocal and extended depth-of-focus lenses: a scoping review. BMC Ophthalmol. 2023;23(1):101. 20. Sabur H, Unsal U. Visual outcomes of non-diffractive extended depth-of-focus and enhanced monofocal intraocular lenses: a case-control study. Eur J Ophthalmol. 2023;33(1):262-8. 21. Lee JH, Moon SY, Chung HS, et al. Clinical outcomes of a monofocal intraocular lens with enhanced intermediate function compared with an extended depth-of-focus intraocular lens. J Cataract Refract Surg. 2022;48:61-6. 22. Jeon YJ, Yoon Y, Kim T, Koh K. Comparison between an intraocular lens with extended depth-of-focus (Tecnis Symfony ZXR00) and a new monofocal intraocular lens with enhanced intermediate vision (Tecnis Eyhance ICB00). Asia Pac J Ophthalmol. 2021;10:542-7. 23. Villegas EA, Alcon E, Mirabet S, et al. Extended depth of focus with induced spherical aberration in light-adjustable intraocular lenses. Amer J Ophthalmol. 2014;157(1): 142-9. 24. Wong JR, Folden DV, Wandling GR, et al. Visual outcomes of a second-generation, enhanced UV protected light adjustable lens in cataract patients with previous LASIK and/or PRK. Clin Ophthalmol. 2023;17:3379-87. 25. Folden DV, Wong, JR. Visual outcomes of an enhanced UV protected light adjustable lens using a novel co-managed, open-access methodology. Clin Ophthalmol. 2022;16:2413-20. 26. Puig M, Salem Y, Morrow M, Waldman C. Comparison of outcomes of trifocal and extended depth-of-focus intraocular lenses. Invest Ophthalmol Vis Sci. 2022;63:1706-F0024. 27. Zhong Y, Wang K, Yu X, et al. Comparison of trifocal or hybrid multifocal extended depth-of-focus intraocular lenses: a systematic review and meta-analysis. Sci Rep. 2021;11(1):6699. 28. Karam, M, Alkhowaiter N, Alkhabbaz A, et al. Extended depth-of-focus versus trifocal for intraocular lens implantation: an updated systematic review and meta-analysis. Amer J Ophthalmol. 2023;251(5). |

