Annual Retina ReportCheck out the other feature articles in this month's report:Reconsider Your Approach to Diabetic Retinopathy Sizing Up Geographic Atrophy Setting Patient Expectations for Anti-VEGF Therapy Tracing Clues Back to Retinitis Pigmentosa |
Decreased vision is one of the cardinal concerns that sends patients to our offices. Myriad conditions can be behind reports of failing vision, including numerous macular pathologies. Optometrists must be proficient in recognizing macular disease during the ocular examination and in using the latest diagnostic technology to do so.
Although of great visual importance, the macula only encompasses approximately 5.5mm of the central retina.1 A thorough macular assessment is an essential part of a comprehensive or problem-oriented eye exam and can often reveal the underlying etiology of patient’s vision loss. This article addresses several exam elements that may be crucial in the diagnosis of macular pathology, beginning with a targeted history, the use of everyday slit lamp techniques, the benefits of various condensing lenses options and, finally, the use of diagnostic imaging, including photographic techniques and OCT.
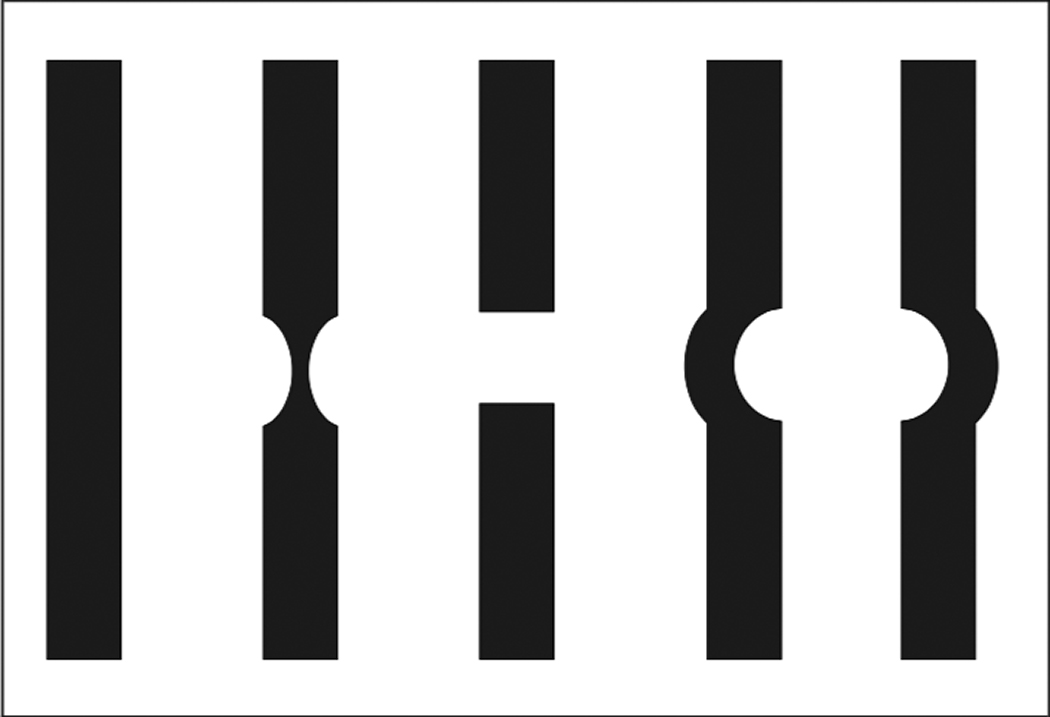 |
| Fig. 1. The WASBT is a good low-tech tool to help distinguish between full-thickness macular holes and pseudo-holes. Patients can describe one of these slit beam options after testing.16 Click image to enlarge. |
Ask Better Questions
A chief complaint and thorough history are integral to an optometric examination and can go a long way toward revealing a macular problem. Symptoms associated with macular disease include blurred vision, loss of central vision or central scotomas, distorted vision or metamorphopsia, loss of color vision, perception of shadows and images that could be fixed or floating, and alteration of image size. Each of these symptoms may suggest a specific form of macular pathology; however, these same symptoms may be caused by other posterior segment pathology such as optic nerve involvement. Asking specific questions about the quality of vision loss may lead us to a better physical examination in search of the actual cause.
Clinicians must ask the right questions to understand, for example, whether the patient is trying to describe a relative scotoma (decreased detail) or absolute scotoma (blacked out or loss of detail). A visual distortion described as an alteration of the image shape is likely a metamorphopsia, while decreased image size is more likely micropsia (caused by foveal cone spreading) and increased image size often indicates macropsia (caused by foveal cone crowding).1
Another important history element is the patient’s systemic diseases and treatment therapies. Many systemic medications are linked with macular complications (Table 1). A history of past ocular surgeries or injuries is important as well. For example, a patient who reports reduced vision after undergoing a recent cataract extraction should prompt the clinician to rule out cystoid macular edema, as it may occur in 1% to 3% of uncomplicated cataract surgeries.2 Additionally, trauma to the head, eyes or body can yield macular manifestations, such as in cases of Purtscher retinopathy, commotio retinae, choroidal rupture, valsalva maculopathy or other posterior pathologies.1,3,4 A history of laser exposure, welding and sun gazing, for example, can cause macula lesions and holes.5-7
Several comorbidities are associated with macular findings, and they should prompt clinicians to take a closer look at the macula. For example, serous detachments develop in about 45% of patients with non-central disc pits.1 Myopic degeneration can manifest as a macular hole, Förster-Fuchs spot, choroidal neovascular membrane, subretinal ‘coin’ hemorrhages and geographic atrophy.1
Diabetes as well as vascular, infectious, retinal and inflammatory diseases can present in the macula. In addition, many systemic conditions can have similar macular findings. For example, non-ischemic central retinal vein occlusion and diabetic retinopathy can each present with “dot-blot” hemorrhages in all four quadrants by the macula and macular edema, so looking at the bigger picture is important.8,9 Macular star is another example of a finding that can have several causes, such as syphilis, cat-scratch disease, severe hypertension retinopathy, cytomegalovirus retinitis posterior hyaloid detachment, non-arteritic anterior ischemic optic neuropathy (incomplete macular star) and Lyme disease.1,10-12
Careful questioning of hereditary fundus dystrophies, such as age-related macular degeneration, can direct your attention to examine the macula closely as well.
Table 1. Medications and the MaculaThese drugs are known to cause damage to the RPE and photoreceptor complex:1
Myriad other drugs are associated with macular damage, including the following reports:
Other medications that have macular manifestations are not available in the United States or present with findings (i.e., cotton-wool spots, hemes) similar to other retinopathies.
|
Lenses: Choose Your Weapon
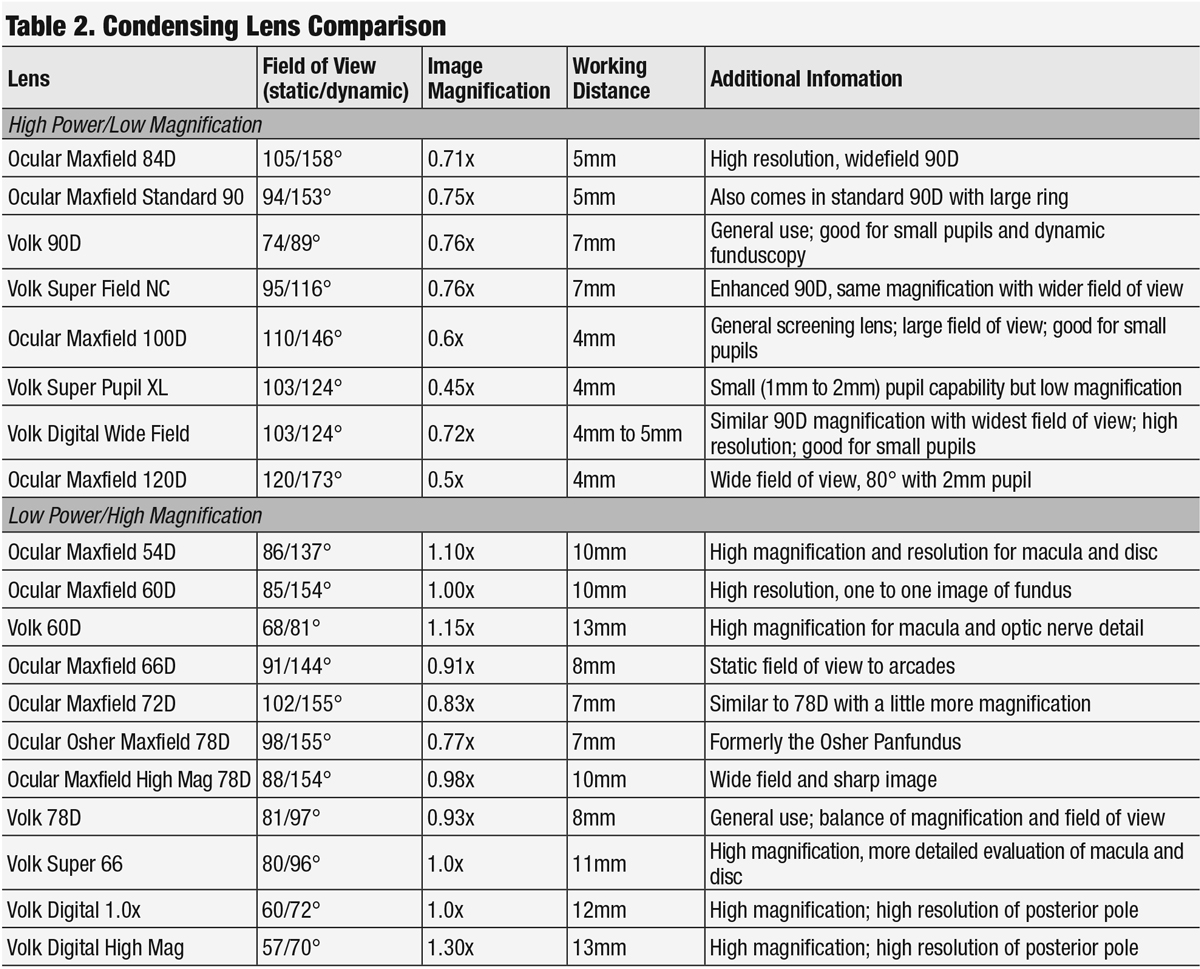
An extensive examination and detection of macular abnormalities requires good magnification and resolution with a condensing lens. Clinicians can have a macula and optic nerve lens for specific evaluations. Though 90D and 78D lenses remain the standard for examining the macula and posterior segment with a slit lamp, a variety of other lenses may also be useful, each with their own benefits (Table 2). Some important things to consider are magnification, dioptric power, field of view, working distance and lens size.
When choosing a lens with higher magnification, remember that as the image size is larger, the field of view is reduced. As such, it may be beneficial to initially perform a gross examination with a lens of lower magnification and then use a higher-powered lens to better see a specific area. In addition, increasing the magnification will decrease the image resolution. The lower the dioptric power of a lenses, the higher the magnification. The higher the dioptric power, the more it converges light. Thus, higher dioptric-powered lenses also require a closer working distance because of the convergence, and closer working distances can induce fogging from patient’s eyes or oil from their lashes.
Because higher-powered lenses are easier to use with small pupils, the 90D is a great choice for undilated exams or for patients with poor pupillary dilation, though the view may be limited based on the aperture size of the pupil, hence the significant benefit of a dilated exam.
Some of the newer lenses can offer the best of both worlds; for instance, an enhanced 78D lens has a wider field of view, higher magnification and larger working distance, and other lenses can provide high resolution, such as the Ocular Maxfield High Mag 78D, and high magnification, such as the Volk Digital 1.0x.
Another non-contact slit lamp lens to consider is the Volk Digital High Mag, which provides high resolution and highest magnification for detailed views of the macula and optic nerve. Macular contact lenses, such as Haag Streit’s 901 fundus contact lens, can provide direct views of the macula and vitreous cavity as far out as 30˚, with fewer issues such as fogging and makeup or oil from the patient’s eye lashes smearing the posterior part of the lens. However, the patient will require topical anesthetic for this procedure. A standard gonioscopy lens can also provide a great view of the posterior pole without an inverted image, all while checking for neovascularization of the angle in diabetes patients.
Red-Free Filter: Seeing Green
Originally, red-free illumination was used to evaluate the peripapillary retinal nerve fiber layer (RNFL) for suspected optic neuropathies.13 The red-free filter (RFF) comes with most slit lamps, ophthalmoscopes and binocular indirect ophthalmoscopes. RFF is also used with fluorescein angiography and fundus photo-graphy. The green light is absorbed by the RPE, which can distinguish between the location of a lesion within certain retinal layers.14 Findings deeper than the RPE tend to disappear, while those in front of the RPE tend to stay.
Blood appears darker with RFF, creating enhanced contrast between blood vessels and hemorrhages, and the retinal background.14 For example, the RFF can make hemorrhages appear darker, which can give a clinician more confidence identifying intraretinal microvascular abnormalities or questionable small hemorrhages. RFF can be a great tool to screen for subtle retinopathy in diabetics.
Other uses of RFF include cystoid macular edema, in which the fluid-filled area in the macula appears light gray. Epiretinal membrane striae appear more noticeable since the RPE disappears with RFF and can take away some glare from white light. In addition, central serous choroidopathy and pigment epithelial detachment borders can look more defined.
The filter can be used to look for a cuff of subretinal fluid around a macular hole. Retinal nevi will disappear or become lighter/gray with RFF, while choroidal hypertrophy of the RPE will remain visible. Drusen, exudates and cotton-wool spots in the macula all show as increased contrast with RFF.
However, the tool isn’t perfect, and the overall views of the macula and retina are darker because of the green light compared with white light. Luckily, increasing the illumination helps and does not seem to bother patients much.
 |
| Click table to enlarge. |
The Poor Person's OCT
The Watzke-Allen Slit Beam Test (WASBT) is a subjective visual function assessment that can determine the loss or distortion of foveal photoreceptors.13 Historically, the WASBT was used for evaluation of macular disease.14 We now use this test for distinguishing between full-thickness macular holes and pseudo-holes. The advantages of the WASBT is its availability, affordability, and the ability to use it through relatively hazy media, which can help make up for the limitations of spectral-domain OCT.15 One study found that WASBT was more useful than SD-OCT initially following macular hole closure of a gas-filled eye due to light reflex and media opacity.15
The WASBT is best performed on a dilated eye with the slit lamp. A slit beam of approximately 100µm is placed on the center of the macula with a non-contact slit lamp (or macula contact) lens.16 The beam is tested in the vertical and then horizontal orientations. The patient is asked to look at the middle of the beam and remember any details regarding the slit beam, such as whether the beam is regular in outline or if it is distorted. Often, patients are asked to draw a representation of the slit beam after the exam.17 The patient could also identify the slit beam characteristics on a diagram (Figure 1).14
A broken light beam is considered a positive Watzke sign. One study suggests that thinning of the slit beam can also be a sign of a full-thickness macular hole and a positive Watzke sign.16 Other researchers found a positive Watzke signs in 91% of patients with medium and large full-thickness macular holes and 67% of patients with a small full-thickness macular hole, vitreomacular traction and lamellar macular holes.18 Indication for treatment sensitivity was 93%, but 33% for specificity with the WASBT.18
Any negative sign, however, with a presumed macular hole would still warrant further evaluation by OCT, if available. Regardless, the WASBT remains a useful tool in practice, as not all clinicians have access to advanced technology in their office. Even as a simple screening tool, WASBT can help lead the clinician to a presumed diagnosis.
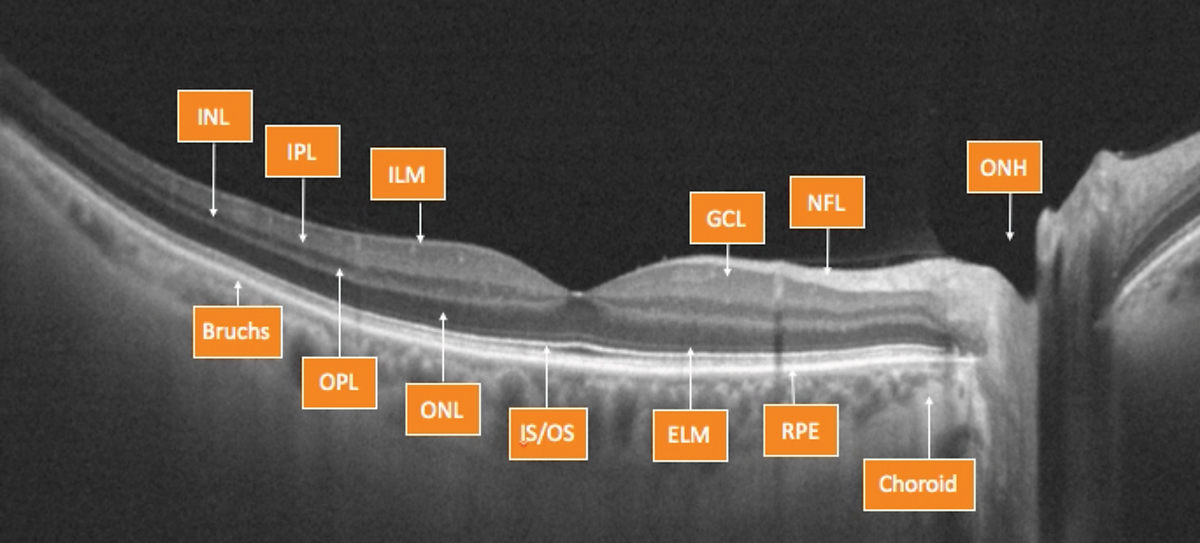 |
Fig. 2. OCT can help clinicians evaluate the retina, layer by layer. Photo by Julie Rodman, OD. Click image to enlarge. |
High-tech Evaluation
Evaluating the different macular layers can be challenging during a fundus examination, as the tissue may appear normal. Though other noted techniques may help the clinician, a more in-depth analysis is often warranted. OCT is one of the most useful devices that have come to market to assist the evaluation of these patients.
As many macular conditions affect specific layers of the retina, the ability to image these layers individually is crucial (Figure 2). The ability to differentiate layers can help clinicians determine between various edematous conditions that may appear similar, but have different etiologies revealed by the location of the changes—warranting individualized treatment approaches.19
OCT can also assist the clinician in assessing the vitreoretinal interface. This becomes beneficial when evaluating for epiretinal membranes and vitreomacular traction (VMT), as even subtle changes can cause visual symptoms. Clinicians should consider imaging with OCT to evaluate for VMT when there is an ERM with striations radiating into the macula or a macula with non-uniform pigmentation, noted funduscopically, often associated with an adjacent ERM.
The advent of OCT has the additional purpose of patient education. The images are excellent tools to help patients understand their ocular status. It also allows for collaboration with other providers, such as retina specialists.
OCT is also a means to measure the RNFL to assist in the diagnosis and management of glaucoma. Studies show that there is also benefit in evaluating the macula to gain information regarding glaucoma. Thinning in the three most inner layers (macular RNFL, macular ganglion cell layer and macular inner plexiform layer) can be indicative of glaucomatous changes and may be beneficial in glaucoma diagnosis and management.20
More recent OCT advancements, such as OCT angiography, can capture blood movement through the vasculature, allowing for computer assessment that results in a simulated angiography, without the complications associated with more invasive fluorescein angiography.21
OCT is not the only imaging tool proven beneficial in macular evaluations. Fundus autofluorescence (FAF) can help clinicians evaluate the macula for possible defects. This technology is readily available on many imaging devices, including posterior segment cameras and OCT. FAF emits a blue light that is absorbed in molecules found within retinal tissue. The excited molecules emit return light the device then evaluates for certain compounds in varying levels. As an example, FAF can reveal lipofuscin density changes often found in certain inherited retinal conditions, macular degeneration and choroidal melanomas.
Clinicians can also use FAF to evaluate the macular region in patients taking hydroxychloroquine, and, in severe disease, the macula may develop the classic bull’s eye changes and continue to lose reflectivity, resulting in a dark macula.22
Scanning laser ophthalmoscopy is another effective tool for evaluating the macula. As with FAF, this technology is often integrated into other devices, mainly OCTs, but may also be stand-alone. It can be enhanced with adaptive optics that provide more in-depth scanning capabilities. In essence this technology uses a scanning laser and software to create high-resolution images.23 These images can help the clinician assess changes in the tissue appearances.
Several examination techniques, some of which have been in the clinician’s toolbox for years, are key to a good macular exam. These techniques can be augmented by the use of diagnostic lenses. With the advent of imaging devices, the macular exam can be more extensive and thorough. OCT is a great tool that can yield a closer look at each layer involved. OCT-A, FAF and SLO are also helpful technologies when evaluating the macula. By meshing these techniques and technology, clinicians have more at their disposal than ever before to appropriately evaluate the macula and choose the best course of action.
Dr. Canizales is an independent contractor providing eye care in Ophthalmology and Optometry private practices in North Carolina.
Dr. Suhr is the chief of optometry at the Corporal Michael J. Crescenz Vetereans Affairs Center in Philadelphia, Pa. He is a Fellow of the Optometric Retina Society.
1. Kanski J, Milewski S. Diseases of the macula: a practical approach. 1st ed. London: Mosby; 2002:1-18. 2. Samiy N, Foster CS. The role of nonsteroidal antiinflammatory drugs in ocular inflammation. Int Ophthalmol Clin. 1996;36(1):195-206. 3. Sekeryapan Gediz B. Acute macular neuroretinopathy in purtscher retinopathy. Turk J Ophthalmol. 2020;50(2):123-26. 4. Scott R. The injured eye. Philos trans R Soc Lond B Biol Sci. 2011;366(1562):251-60. 5. Suhr CL, Buffano RM, Sellers A. The use of optical coherence tomography to aid in diagnosing solar maculopathy. Optometry. 2011;82:481-84. 6. Birtel J, Harmening WM, Krohne TU, et al. Retinal injury following laser pointer exposure: A systemic review and case series. Dtsch Arztebl Int. 2017;114(49):831-37. 7. Mahindraker A, Toshniwal S, Doongerwala MI, Anthony H. Spectralis optical coherence tomography findings in Welder’s maculopathy. Indian J Ophthalmol. 2013;61(5):238-40. 8. Hayreh SS, Podhajsky PA, Zimmerman MB. Natural history of visual outcome in central retinal vein occlusion. Ophthalmology. 2011;118(1):119-33.e2. 9. Sivaprasad S, Pearce E. The unmet need for better risk stratification of non-proliferative diabetic retinopathy. Diabet Med. 2019;36(4):424-33. 10. Narayan SK, Kaliaperumal S, Srinivasan R. Neuroretinitis, a great mimicker. Ann Indian Acad Neurol. 2008;11(2):109-13. 11. Galvez-Ruiz A. Macular star formation in diabetic patients with non-arteritic anterior ischemic optic neuropathy (NA-AION). Saudi J Ophthalmol. 2015;29(1):71-75. 12. Akura J, Ikeda T, Sato K, Ikeda N. Macular star associated with posterior hyaloid detachment. Acta Ophthalmol Scand. 2001;79(3):317-8. 13. Miller N, George T. Monochromatic (red-free) photography and ophthalmoscopy of the peripapillary retinal nerve fiber layer. Invest Ophtalmol Vis Sci. 1978;17 (11):1121-24. 14. Walling P, Dinardo A. The lost arts of optometry, part two: put the ‘fun’ back in funduscopy. Rev Optom. 2013;150(9):58-66. 15. Yamakiri K, Sakamoto T. Early diagnosis of macular hole closure of a gas-filled eye with Watzke-Allen slit beam test and spectral domain optical coherence tomography. Retina. 2012;32(4):767-72. 16. Tanner V, Williamson T. Watzke-Allen Slit Beam Test in macular holes confirmed by optical coherence tomography. Arch Ophthalmic. 2000;118:1059-63. 17. Watzke RC, Allen L. Subjective slitbeam sign for macular disease. Am J Ophthalmol. 1969;68(3):449-53. 18. Fischer C, Callizo J, Wetzel E, et al. Importance of Watzke-Allen Test in Diagnostics and staging of macular holes. Ophthalmologe. 2016;113(2):152-5. 19. Trichonas G, Kaiser PK. Optical coherence tomography imaging of macular oedema. Br J Ophthalmol. 2014;98(Suppl 2):ii24–ii29. 20. Chua J, et al. Diagnostic ability of individual macular layers by spectral-domain optical coherence tomography in different stages of glaucoma. Ophthalmol Glaucoma. April 21, 2020. [Epub ahead of print]. 21. Hagag AM, Gao SS, Jia Y, Huang D. Optical coherence tomography angiography: Technical principles and clinical applications in ophthalmology. Taiwan J Ophthalmol. 2017;7(3):115-29. 22. Yung M, Klufas MA, Sarraf D. Clinical applications of fundus autofluorescence in retinal disease. Int J Retin Vitr. 2016;2:12. 23. Joeres S, Jones SM, Chen DC, et al. retinal imaging with adaptive optics scanning laser ophthalmoscopy in unexplained central ring scotoma. Arch Ophthalmol. 2008;126(4):543-47. |

