 |
An 82-year-old man presented with a moderately painful left eye for the previous two weeks. He was diabetic and hypertensive and had been diagnosed with open-angle glaucoma in both eyes nearly two years earlier. His right eye was in the advanced stage and he had moderate nonproliferative diabetic retinopathy (DR) in each eye. He was prescribed a prostaglandin analog for glaucoma and reappointed for ongoing care, but was lost to follow-up until now.
At this visit, he was 20/25 OD and bare light perception OS. He was bilaterally pseudophakic with posterior chamber intraocular lenses in each eye. He reported still using latanoprost. He had moderate glaucomatous optic nerve damage in the right eye with a small amount of neovascularization of the disc and DR elsewhere. His intraocular pressures (IOP) were 23mm Hg OD and 62mm Hg OS. His left eye manifested central microcystic corneal edema, florid iris neovascularization and hyphema. Gonioscopically, his right angle was open to at least scleral spur while his left angle was closed with peripheral anterior synechiae (PAS) for 180˚, with hyphema and angle neovascularization for the remaining part of the angle. Due to corneal edema, circulating red blood cells in the anterior chamber and a poor dilation, we could not see the left fundus.
Clearly, this was an acutely urgent case of neovascular glaucoma (NVG).
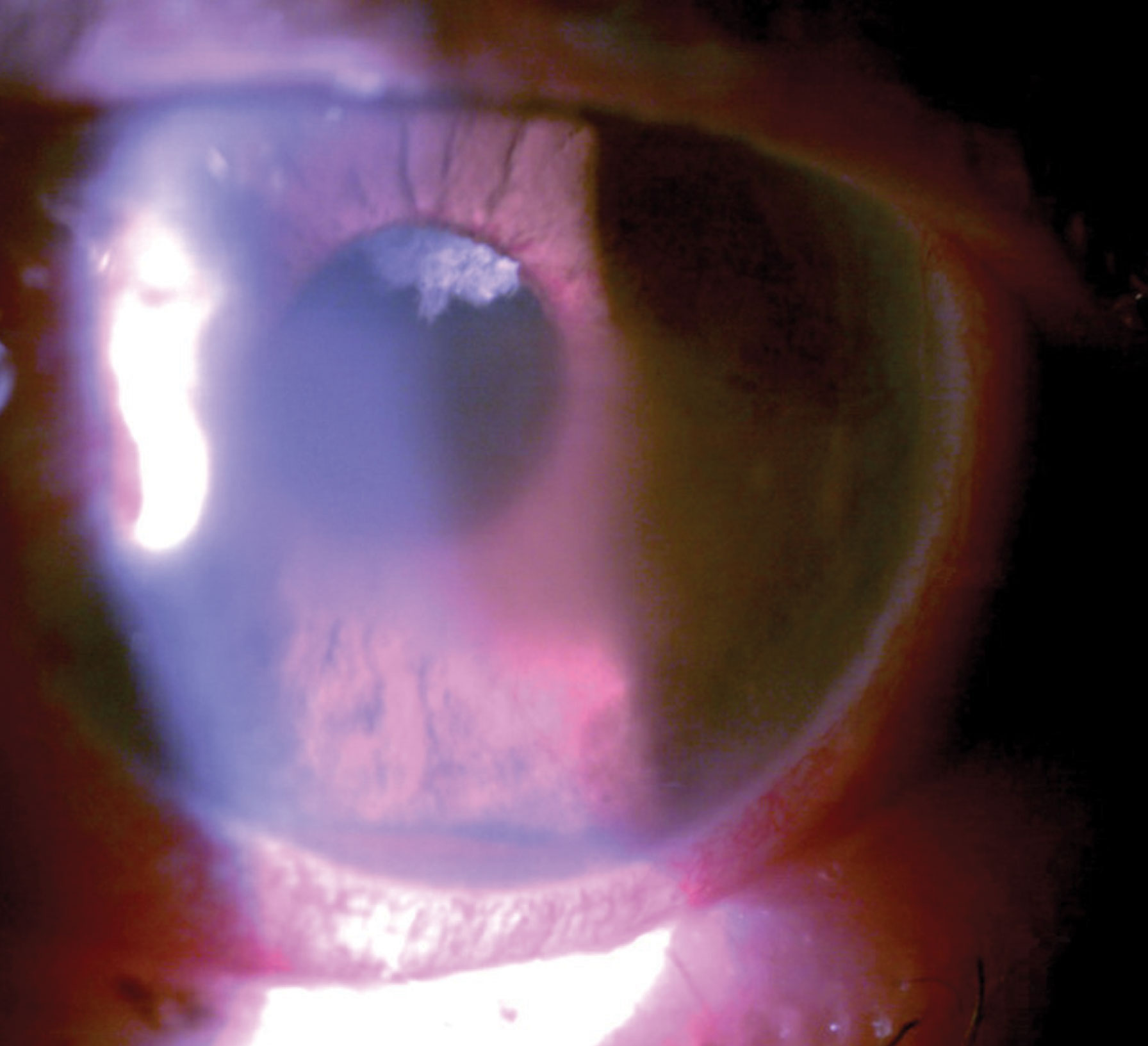 |
| This patient had pain with vision loss and profound pathological findings such as hyphema, microcystic edema and peripupillary iris neovascularization. Click image to enlarge. |
Discussion
Patients with NVG typically present with a chronically red, painful eye, which often has significant vision loss. The majority of patients’ presenting acuity will be below 20/200.1-3 They often have significant concurrent vascular disease, such as diabetes, hypertension or giant cell arteritis (GCA)— diabetes is the most common precipitating cause of NVG.3-8 Patients are also likely to have an antecedent history of a retinal vessel occlusion, carotid artery disease, chronic retinal detachment or advanced DR.5,8
These patients will have visible neovascularization of the iris and angle. The patient will typically have significant corneal edema, anterior segment inflammation, anterior chamber cell and flare reaction. They’ll usually have elevated IOP, often exceeding 60mm Hg.1,9,10 Gonioscopically, there will be total or near-total angle closure with massive areas of PAS and neovascularization of the angle. In early cases, microhyphema may be seen gonioscopically. Funduscopically, there will often be evidence of retinal vessel occlusion (either artery or vein), DR, ocular ischemic syndrome or another condition stimulating retinal ischemia.
Retinal hypoxia induces vascular endothelial growth factor (VEGF) to act upon healthy endothelial cells of viable capillaries to stimulate the formation of a fragile new plexus of vessels (neovascularization).11-16 In cases of extreme retinal hypoxia—such as ischemic central retinal vein occlusion—very few retinal capillaries are viable. In that instance, researchers theorize that VEGF can diffuse forward to the nearest area of viable capillaries, namely the posterior iris. Neovascularization buds off of the capillaries of the posterior iris, grows along the posterior iris, through the pupil, along the anterior surface of the iris and then into the angle. Once in the angle, the neovascularization, along with attendant fibrovascular support membrane, acts to both physically block the angle as well as bridge the angle and physically pull the iris and cornea into apposition. This blocks the trabecular meshwork with progressive PAS.17
Management
Neovascular glaucoma requires prompt and aggressive therapy that involves IOP and inflammation control as well as management of retinal ischemia and any precipitating conditions. Upon first presentation, prescribe a strong cycloplegic such as atropine 1% BID as well as a topical steroid such as prednisolone acetate 1% or difluprednate 0.05% QID.10 The fact that the angle may be closed with PAS does not preclude pharmacologic mydriasis from atropine. Neither does the elevated IOP disqualify steroid use. This will greatly add to patient comfort. Aqueous suppressants in the form of beta blockers, carbonic anhydrase inhibitors and alpha adrenergic agonists may be used to temporarily reduce IOP.10 However, chronic medical therapy is not indicated for neovascular glaucoma. Aqueous suppressants will temporize IOP and lead to a false sense of security, as the neovascular process will continue with further angle closure.18
Management of anterior segment neovascularization and NVG involves eradication of the vessels. This is best accomplished with pan-retinal photocoagulation (PRP) to destroy ischemic retina, minimize oxygen demand of the eye, and reduce the amount of VEGF being released.19-22 PRP tends to effectively cause regression and involution of anterior segment neovascularization in approximately 60% of cases.17 This may also reduce IOP as well as induce vessel regression.
While PRP is the most definitive treatment for the neovascularization causing NVG, the advent and use of antiangiogenic drugs has proven a valuable adjunct. Intravitreal injectable agents such as bevacizumab, ranibizumab and aflibercept have been demonstrated through numerous reports, both controlled studies and case series, to cause prompt and thorough regression of anterior segment neovascularization in NVG.23-28 Regression can be significant and occur within a period as short as one day following injection.26 Neovascularization can recur following antiangiogenic injection if the causative factor is unaddressed. For that reason, antiangiogenic therapy should only be considered a valuable adjunct along with laser photoablation in the management of NVG.29,30
Medical therapy following PRP is common, though frequently insufficient to manage the glaucoma. Often, IOP lowering surgical methods are required. Trabeculectomy with antimetabolite adjuncts have effectively managed the elevated IOP in NVG.5 Often, the use of drainage devices are necessary to optimize surgical outcomes.31, 32
This patient’s NVG clearly resulted from ischemic retinal disease. He was prescribed atropine 1% BID, prednisolone acetate 1% QID, brinzolamide/brimonidine fixed combination TID in the left eye and latanoprost QHS OU and scheduled for definitive treatment with a retinal specialist. Upon meeting with the retina specialist approximately two weeks later, his eye pain had disappeared, his IOP lowered to 24mm Hg OS and his cornea had cleared enough to allow fundus examination. He still had an iris neovascularization, but the hyphema had cleared. Fundus examination of the right eye revealed end stage glaucomatous atrophy and extensive retinal hemorrhaging. Fluorescein angiography demonstrated poor flow into the left eye and a combined central retinal artery and vein occlusion. He received an anti-VEGF injection and was scheduled for PRP.
| 1. Kuang TM, Liu CJ, Chou CK, et al. Clinical experience in the management of neovascular glaucoma. J Chin Med Assoc. 2004;67(3):131-5. 2. Nabili S, Kirkness CM. Trans-scleral diode laser cyclophoto-coagulation in the treatment of diabetic neovascular glaucoma. Eye. 2004;18(4):352-6. 3. Al-Shamsi HN, Dueker DK, Nowilaty SR, Al-Shahwan SA. Neovascular glaucoma at king khaled eye specialist hospital - etiologic considerations. Middle East Afr J Ophthalmol. 2009;16(1):15-9. 4. Lawrence PF, Oderich GS. Ophthalmologic findings as predictors of carotid artery disease. Vasc Endovascular Surg. 2002;36(6):415-24. 5. Mandal AK, Majji AB, Mandal SP, et al. Mitomycin-C-augmented trabeculectomy for neovascular glaucoma. A preliminary report. Indian J Ophthalmol. 2002;50(4):287-93. 6. Hamanaka T, Akabane N, Yajima T, et al. Retinal ischemia and angle neovascularization in proliferative diabetic retinopathy. Am J Ophthalmol. 2001;132(5):648-58. 7. Chen KJ, Chen SN, Kao LY, et al. Ocular ischemic syndrome. Chang Gung Med J. 2001;24(8):483-91. 8. Detry-Morel M. Neovascular glaucoma in the diabetic patient. Bull Soc Belge Ophtalmol. 1995;256:133-41. 9. Shazly TA, Latina MA. Neovascular glaucoma: etiology, diagnosis and prognosis. Semin Ophthalmol. 2009;24(2):113-21. 10. Löffler KU. Neovascular glaucoma: aetiology, pathogenesis and treatment. Ophthalmologe. 2006;103(12):1057-63 11. Kozawa T, Sone H, Okuda Y, et al. Vascular endothelial growth factor levels in the aqueous and serum in diabetic retinopathy with or without neovascular glaucoma. Nippon Ganka Gakkai Zasshi. 1998;102(11):731-8. 12. Pe’er J, Folberg R, Itin A, et al. Vascular endothelial growth factor upregulation in human central retinal vein occlusion. Ophthalmology. 1998;105(3):412-6. 13. Tripathi RC, Li J, Tripathi BJ, et al. Increased level of vascular endothelial growth factor in aqueous humor of patients with neovascular glaucoma. Ophthalmology. 1998;105(2):232-7. 14. Tolentino MJ, Miller JW, Gragoudas ES, et al. Vascular endothelial growth factor is sufficient to produce iris neovascularization and neovascular glaucoma in a nonhuman primate. Arch Ophthalmol. 1996;114(8):964-70. 15. Hu DN, Ritch R, Liebmann J, et al. Vascular endothelial growth factor is increased in aqueous humor of glaucomatous eyes. J Glaucoma. 2002;11(5):406-10. 16. Scholl S, Kirchhof J, Augustin AJ. Antivascular endothelial growth factors in anterior segment diseases. Dev Ophthalmol. 2010;46:133-9. 17. Hamard P, Baudouin C. Consensus on neovascular glaucoma. J Fr Ophtalmol. 2000;23(3):289-94. 18. Sivak-Callcott JA, O’Day DM, Gass JD, et al. Evidence-based recommendations for the diagnosis and treatment of neovascular glaucoma. Ophthalmology. 2001;108(10):1767-76. 19. Brooks AM, Gillies WE.The development and management of neovascular glaucoma. Aust N Z J Ophthalmol. 1990;18(2):179-85. 20. Cashwell LF, Marks WP. Panretinal photocoagulation in the management of neovascular glaucoma. South Med J. 1988;81(11):1364-8. 21. Stefaniotou M, Paschides CA, Psilas K. Panretinal cryopexy for the management of neovascularization of the iris. Ophthalmologica. 1995;209(3):141-4. 22. Lee SC, Kim GO, Kim DH, et al. Endoscopic laser photocoagulation for management of neovascular glaucoma. Yonsei Med J. 2000;41(4):445-9. 23. Lee SJ, Lee JJ, Kim SY, Kim SD. Intravitreal bevacizumab (Avastin) treatment of neovascular glaucoma in ocular ischemic syndrome. Korean J Ophthalmol. 2009;23(2):132-4. 24. Yazdani S, Hendi K, Pakravan M, et al. Intravitreal bevacizumab for neovascular glaucoma: a randomized controlled trial. J Glaucoma. 2009;18(8):632-7. 25. Beutel J, Peters S, Lüke M, et al. Bevacizumab as adjuvant for neovascular glaucoma. Acta Ophthalmol. 2010;88(1):103-9. 26. Batioğlu F, Astam N, Ozmert E. Rapid improvement of retinal and iris neovascularization after a single intravitreal bevacizumab injection in a patient with central retinal vein occlusion and neovascular glaucoma. Int Ophthalmol. 2008;28(1):59-61. 27. Katsanos A, Gorgoli K, Mikropoulos DG, et al Assessing the role of ranibizumab in improving the outcome of glaucoma filtering surgery and neovascular glaucoma. Expert Opin Biol Ther. 2018;18(6):719-24. 28. Andrés-Guerrero V, Perucho-González L, García-Feijoo J, et al. Current perspectives on the use of anti-VEGF drugs as adjuvant therapy in glaucoma. Adv Ther. 2017;34(2):378-95. 29. Hasanreisoglu M, Weinberger D, Mimouni K, et al. Intravitreal bevacizumab as an adjunct treatment for neovascular glaucoma. Eur J Ophthalmol. 2009;19(4):607-12. 30. Ciftci S, Sakalar YB, Unlu K, et al. Intravitreal bevacizumab combined with panretinal photocoagulation in the treatment of open angle neovascular glaucoma. Eur J Ophthalmol. 2009;19(6):1028-33. 31. Alkawas AA, Shahien EA, Hussein AM. Management of neovascular glaucoma with panretinal photocoagulation, intravitreal bevacizumab, and subsequent trabeculectomy with mitomycin C. J Glaucoma. 2010 Feb 22. [Epub ahead of print] 32. Eid TM, Radwan A, el-Manawy W, el-Hawary I. Intravitreal bevacizumab and aqueous shunting surgery for neovascular glaucoma: safety and efficacy. Can J Ophthalmol. 2009;44(4):451-6. |

