We spend the majority of our careers making diagnoses on other people. But occasionally, we have to turn the tables and use our clinical acumen to diagnose personal medical situations. This happened to me recently.

First, a bit of history. Previously, I was a high myope with a refractive error of approximately -10.00D O.U. (with some negligible cylinder). Like many patients tired of thick glasses and intolerant of contact lenses, I rode the wave of refractive surgery. Approximately 13 years ago, I underwent LASIK vision correction. Stanley Braverman, M.D., of Hallandale Beach, Fla., did a fantastic job, resulting in a nearly emmetropic outcome with outstanding visual acuity and no complications. Thus, I became a functional emmetrope for the first time. I spent the ensuing years free of all visual correction and was completely happy.
Then, a few years ago, I started to wear a minimal myopic correction for night driving. Next, I began to wear the spectacles a little more frequently in other dim-light situations, such as cocktail parties and when delivering lectures. Of course, I changed the prescription slightly every year. A mild bit of regression was expected, and still I was extremely happy with my LASIK surgery.
After a while, however, I discovered that not only was the myopic prescription in my right eye increasing, but also my best-corrected visual acuity was decreasing. Initially, my best-corrected acuity in my right eye dropped to 20/25 from 20/15. However, the left eye still remained unchanged in refraction and I could see 20/15 with that eye. As such, I wasn’t terribly bothered by the slight blur in the right eye.
Then, over the next 18 months, my best-corrected visual acuity in the right eye dropped first to 20/30, then to 20/40. Then my vision dropped further to 20/50 over a relatively short period of time. I developed monocular diplopia in my right eye (which isn’t nearly as troublesome as you might think). At that time, in early 2010, I embarked on a search for the cause. While I couldn’t be refracted to my satisfaction, I pinholed to a sharp 20/20 O.D. Anterior segment biomicroscopy was completely normal. My crystalline lenses were graded as clear and virtually symmetrical by several close and trusted colleagues. Some mentioned a bit of haze, but nothing commensurate with my vision.
Corneal topography showed a pristine post-LASIK pattern. There was no corneal thinning or endothelial disease. There was no irregular astigmatism, and a rigid gas permeable contact lens with over-refraction gave no better vision. Threshold perimetry was full and optical coherence tomography revealed normal macular architecture. Pupil testing was normal without afferent defect, and color vision testing was full and symmetrical. I stopped short of ordering an MRI on myself. A retinal specialist proclaimed my fundus to be perfect. Doing my own diagnostic evaluation and working with several colleagues, there was no apparent cause.
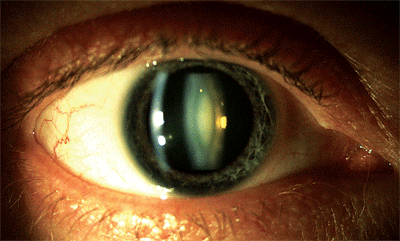
Slit lamp view of my dense, “milky” nuclear cataract. Photo: Lori Vollmer, O.D.
When I reviewed my refractive history, I saw that, in 2008, the prescription in my right eye was -0.75D, then it was -1.50D in 2009 and then it increased to -3.00D in 2010. Finally my refraction in 2011 measured -5.00D, with a best-corrected visual acuity of just 20/70. I had a significant and rapid refractive shift. I still couldn’t be refracted to acceptable acuity (although, with pinhole, my acuity was quite sharp and the monocular diplopia disappeared). I came to the conclusion that, at age 47 (actually likely beginning at age 45), I had developed a visually significant cataract. I couldn’t believe it.
I had the dilated exam repeated and, when using a very thin beam from the biomicroscope, it was now easily seen that I had a dense, central, nuclear opalescence or what is commonly termed “milky” nuclear sclerosis in my right eye with a clear crystalline lens in my left eye.
A Stealthy Cataract
There are numerous types of cataracts seen in clinical practice––from generalized nuclear sclerosis, to cortical changes, to posterior subcapsular cataracts, to many types in between.1-3 One type is “white” nuclear sclerosis, which is often referred to as nuclear opalescence or “milky” nuclear sclerosis. This delineates a unique type of cataract that is not often described in the literature as a distinct clinical entity. This type of cataract apparently has specific and unusual properties and behaviors, including a dramatic myopic shift with significant visual impairment despite an unobstructed view of the fundus. Hence, this type of cataract often goes undiagnosed. Despite the clear view of the fundus, differing refractive indices can produce a bowing effect of the slit beam when examining the retina with a biomicroscope and non-contact fundus lens.
We have all learned that with cataracts, the “view in” should roughly equal the “view out;” in other words, we expect the patient’s visual acuity to be a fair approximation of the examiner’s observed fundus clarity. However, when dealing with the milky nuclear sclerotic cataract, this rule doesn’t apply. The view in is much better than the view out. In my case, a retinal specialist examined my fundus with no viewing difficulties whatsoever, yet my corrected acuity was 20/60 O.D. at that time.
The disparity between acuity and funduscopic view is so great that a local neuro-ophthalmologist remarked that he has seen numerous cases of this type of cataract as these patients are referred to him for unexplained vision loss.
Interestingly, two surgical colleagues have affectionately named this type of lenticular opacification a “stealth cataract.” That is, it develops both quickly and silently and many clinicians don’t even know that it’s there. These colleagues went on to say that they occur commonly in middle-aged, highly myopic males. They also had personally noted a higher incidence in highly myopic individuals that have had LASIK surgery, but agreed that they also occur without the refractive surgery history. Their experience was that the cataract was disproportionate to the patient’s visual acuity, but cataract removal was met with a significant visual recovery.
How Does it Occur?
Throughout my evaluation, I had many conversations about this type of cataract with several surgeons. While some cataract surgeons have noted anecdotally that milky nuclear sclerotic cataracts occur after LASIK surgery, others have not noted this association. When searching the literature, there is virtually no reported association between LASIK surgery and milky nuclear sclerotic cataracts. But, there is a known association between
cataract formation and high myopia.4,5 Most surgeons I spoke with believed this was most likely the association in my case, but they all admitted their suspicions were only speculations. Nobody could explain the unilateral presentation. Several cataract surgeons have told me that they have occasionally observed these cataracts in association with high myopia, post-LASIK or both, but not to the extent that they would consider anything causal. Again, there is very little literature regarding this type of cataract, let alone any proven causative factor.
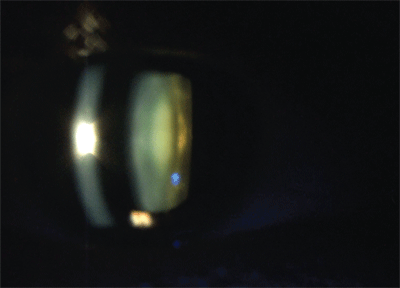
A thin slit beam from the biomicroscope revealed the dense central nuclear opalescence. Photo: Lori Vollmer, O.D.
I’ve never experienced ocular trauma or used topical steroids, and I don’t have any predisposing systemic conditions. I am (or was formerly) a highly myopic, now middle-aged male who had LASIK surgery. Which of these factors, if any, contributed to a unilateral visually significant milky nuclear opalescence beginning at age 45 is unknown. However, it’s important to remember that a type of cataract exists that is characterized by a dense, milky nuclear opalescence, which is out of proportion to the degree of visual disability. It may occur earlier in life and seems more common in middle-aged, highly myopic males. It can easily be missed. The key is to look at the nuclear core with a very thin slit beam on the microscope and to remember that the view in is not consistent with the view out.
In the end, I underwent phacoemulsification with a clear acrylic implant. Alberto Aran, M.D., of Coral Gables, Fla., did such a phenomenal job that my uncorrected visual acuity was 20/20 O.D. six hours after surgery. I used Durezol (difluprednate ophthalmic emulsion 0.05%, Alcon) q.i.d. and was told I had the clinical post-op response at one day that is normally seen at one week (likely due to a combination of this steroid and Dr. Aran’s superb surgical skill). Today, as I write this, I am 20/15 O.D. without correction.
I guess it was just a cataract after all.
Dr. Sowka is a consultant for Alcon, but has no direct financial interest in any of the products mentioned.
1. Chylack LT Jr, Leske MC, McCarthy D, et al. Lens opacities classification system II (LOCS II). Arch Ophthalmol. 1989 Jul;107(7):991-7.
2. Chylack LT Jr, Wolfe JK, Friend J, et al. Quantitating cataract and nuclear brunescence, the Harvard and LOCS systems. Optom Vis Sci. 1993 Nov;70(11):886-95.
3. Chylack LT Jr, Wolfe JK, Singer DM, et al. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993 Jun;111(6):831-6.
4. Younan C, Mitchell P, Cumming RG, et al. Myopia and incident cataract and cataract surgery: The Blue Mountains Eye Study. Invest Ophthalmol Vis Sci. 2002 Dec;43(12):3625-32.
5. Brown NA, Hill AR. Cataract: the relation between myopia and cataract morphology. Br J Ophthalmol. 1987 Jun;71(6):405-14.

