 Within the realm of eye disease, allergy poses a tremendous
opportunity for optometric practice. This condition, quite prevalent in the
population, is a greatly underserved need. Moreover, myriad available topical
ocular medications—e.g., Pataday (olopatadine 0.2%, Alcon), Elestat (epinastine
HCl 0.05%, Allergan), Zaditor (ketitofen fumarate 0.025%, Novartis), Optivar
(azelastine hydrochloride 0.05%, MedPointe) and Alrex (loteprednol etabonate
0.2%, Bausch & Lomb)—render fast, effective relief of symptoms associated
with seasonal allergic conjunctivitis.
Within the realm of eye disease, allergy poses a tremendous
opportunity for optometric practice. This condition, quite prevalent in the
population, is a greatly underserved need. Moreover, myriad available topical
ocular medications—e.g., Pataday (olopatadine 0.2%, Alcon), Elestat (epinastine
HCl 0.05%, Allergan), Zaditor (ketitofen fumarate 0.025%, Novartis), Optivar
(azelastine hydrochloride 0.05%, MedPointe) and Alrex (loteprednol etabonate
0.2%, Bausch & Lomb)—render fast, effective relief of symptoms associated
with seasonal allergic conjunctivitis.
In 2007, however, the ophthalmic community was turned on its ear when one nasal allergy medication, Veramyst (fluticasone furoate, GlaxoSmithKline), was approved with the following information in its package insert: “Veramyst may also help red, itchy and watery eyes in adults and teenagers with seasonal allergic rhinitis.”1
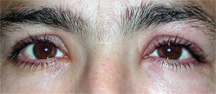 |
| Can ocular allergies be soothed by nasal medications?
|
Believe it or not, the literature clearly supports the use of nasal corticosteroids for the relief of ocular symptoms associated with allergy.2-9 Some of these reports date back more than 15 years, although earlier studies were constructed primarily to evaluate nasal allergy; improvement of ocular symptoms was a secondary, incidental finding.2-4 A 2004 report was one of the first studies specifically designed to determine the efficacy of a once-daily intranasal steroid, Flonase (fluticasone propionate, GlaxoSmithKline), to improve ocular symptoms associated with allergic rhinitis.6 This prospective, placebo-controlled, multi-center trial reflectively evaluated 471 subjects over four weeks for ocular itching, tearing and redness throughout the day. These findings were tallied to create a total ocular symptom score (TOSS) for each patient at days 1, 15 and 29; then, the mean change in TOSS was calculated. According to the study, the overall change from baseline to day 29 was significantly greater for individuals who used Flonase than for subjects in the placebo group (-88.7 vs. -59.9, p<0.001).6
Similar studies have been conducted since with other nasal steroids, including Veramyst and Nasonex (mometasone furoate, Schering Plough).7-10 The results conclusively demonstrate that these preparations can, like Flonase, help to ameliorate the ocular symptoms of allergy in addition to nasal symptoms. Some researchers have even used these findings to argue that the ocular allergic response is predominantly due to active nasal inhalation and accumulation of allergens, rather than simply “passive” contact with the conjunctiva.7,11 And, because as many as 90% of individuals with allergic conjunctivitis also suffer from rhinitis (and vice versa), is it logical to prescribe a nasal corticosteroid preferentially to an ophthalmic allergy drop?11-13
Keep a Clear View
While we’re always interested in simplifying our patients’ therapies and providing the greatest relief from symptoms, we must be careful not to succumb to hype surrounding new products or alternate routes of administration. Although eye drops may not seem as “sexy” as pills, inhalants or injections, they are nonetheless our most direct and effective means of controlling many ocular disorders, particularly keratitis and conjunctivitis.
With regard to ocular allergy, topical agents still appear to offer faster and more complete relief of ocular symptoms than any other form of therapy. In fact, a recent head-to-head study showed that Pataday was statistically and clinically superior to Veramyst in alleviating ocular itching, redness, tearing, chemosis and eyelid swelling associated with allergic conjunctivitis.12 Its authors challenged prior research, which championed nasal corticosteroids as first-line therapy for allergic rhinoconjunctivitis, and they explained that TOSS is not nearly as sensitive a tool as the independent evaluation of signs and symptoms—the standard in the ophthalmic allergy drug industry.11,12 Earlier studies pitting ophthalmic allergy medications against nasal corticosteroids revealed similar findings.13,14
Additionally, there is a small, but definite, risk associated with the use of nasal corticosteroids, a feature not encountered with nonsteroidal ophthalmic allergy medications. Package inserts for Veramyst, Flonase and Nasonex note the potential for development of cataracts, glaucoma and ocular hypertension, and clinical studies corroborate this danger.1,15-19 Rare cases of central serous chorioretinopathy have also been reported in conjunction with nasal corticosteroid use.20 Exercise caution when employing these nasal preparations in the very young, the very old, the immunocompromised, patients with pre-existing glaucoma, and patients utilizing additional immunosuppressant therapies.17
So, what’s the bottom line with regard to managing allergic conjunctivitis? Although there is clear evidence that nasal corticosteroids are beneficial with regard to ocular allergy symptoms, clinical experience suggests that ophthalmic preparations are safer and more effective in achieving rapid and sustained relief. In other words, eye drops should remain our primary mode of treating ocular allergy. Nasal sprays may be prescribed as a secondary means of therapy for our patients who have concurrent rhinitis (state laws permitting). Interestingly, studies have shown that oral medications like Zyrtec (cetirizine, Pfizer) and Claritin (loratadine, Schering Corp.) are actually the least effective modality for managing ocular and nasal symptoms associated with allergy, and can also induce secondary dry eye complaints.13,21 Oral antihistamines should be reserved for those with the most acute, severe reactions or chronic, multisystem allergic disease.
1. GlaxoSmithKline. Veramyst package insert. 2007.
2. Gawchik SM, Lim J. Comparison of intranasal triamcinolone acetonide with oral loratadine in the treatment of seasonal ragweed induced allergic rhinitis. Am J Manag Care. 1997 Jul;3(7):1052-8.
3. Dolovich J, Wong AG, Chodirker WB, et al. Multicenter trial of fluticasone propionate aqueous nasal spray in ragweed allergic rhinitis. Ann Allergy. 1994 Aug;73(2):147-53.
4. Settipane G, Korenblat PE, Winder J, et al. Triamcinolone acetonide aqueous nasal spray in patients with seasonal ragweed allergic rhinitis: a placebo-controlled, double-blind study. Clin Ther. 1995 Mar-Apr;17(2):252-63.
5. DeWester J, Philpot EE, Westlund RE, et al. The efficacy of intranasal fluticasone propionate in the relief of ocular symptoms associated with seasonal allergic rhinitis. Allergy and Asthma Proc. 2003 Sep-Oct;24(5):331-7.
6. Bernstein DI, Levy AL, Hampel FC, et al. Treatment with intranasal fluticasone propionate significantly improves ocular symptoms in patients with seasonal allergic rhinitis. Clin Exp Allergy. 2004 Jun;34(6):952-7.
7. Kaiser HB, Naclerio RM, Given J, et al. Fluticasone furoate nasal spray: A single treatment option for the symptoms of seasonal allergic rhinitis. J Allergy Clin Immunol. 2007 Jun;119(6):1430-7.
8. Fokkens WJ, Jogi R, Reinartz S, et al. Once daily fluticasone furoate nasal spray is effective in seasonal allergic rhinitis caused by grass pollen. Allergy. 2007 Sep;62(9):1078-84.
9. Baroody FM, Shenaq D, DeTineo M, et al. Fluticasone furoate nasal spray reduces the nasal-ocular reflex: a mechanism for the efficacy of topical steroids in controlling allergic eye symptoms. J Allergy Clin Immunol. 2009 Jun;123(6):1342-8.
10. Anolik R, Nathan RA, Schenkel E, et al. Intranasal mometasone furoate alleviates the ocular symptoms associated with seasonal allergic rhinitis: results of a post hoc analysis. Int Arch Allergy Immunol. 2008;147(4):323-30.
11. Naclerio RM, Philpot EE. Is conjunctival allergen challenge a model of seasonal rhinoconjunctivitis? Allergy Asthma Proc. 2009 Mar-Apr;30(2):212-3; author reply 213-4.
12. Rosenwasser LJ, Mahr T, Abelson MB, et al. A comparison of olopatadine 0.2% ophthalmic solution versus fluticasone furoate nasal spray for the treatment of allergic conjunctivitis. Allergy Asthma Proc. 2008 Nov–Dec;29(6):644-53.
13. Spangler DL, Abelson MB, Ober A, Gomes PJ. Randomized, double-masked comparison of olopatadine ophthalmic solution, mometasone furoate monohydrate nasal spray, and fexofenadine hydrochloride tablets using the conjunctival and nasal allergen challenge models. Clin Ther. 2003 Aug;25(8):2245-67.
14. Horak F, Stuebner P, Zieglmayer R, et al. Efficacy and safety of ketotifen eye drops as adjunctive therapy to mometasone nasal spray in subjects with seasonal allergic rhinoconjunctivitis. Clin Drug Invest. 2003;23(9):597-604.
15. GlaxoSmithKline. Flonase package insert. 2004.
16. Schering Corp. Nasonex package insert. 2003.
17. Blaiss MS. Safety considerations of intranasal corticosteroids for the treatment of allergic rhinitis. Allergy Asthma Proc. 2007 Mar-Apr;28(2):145-52.
18. Opatowsky I, Feldman RM, Gross R, et al. Intraocular pressure elevation associated with inhalation and nasal corticosteroids. Ophthalmology. 1995 Feb;102(2):177-9.
19. Bielory L, Blaiss M, Fineman SM, et al. Concerns about intranasal corticosteroids for over-the-counter use: Position statement of the Joint Task Force for the American Academy of Allergy, Asthma and Immunology and the American College of Allergy, Asthma and Immunology. Ann Allergy Asthma Immunol. 2006 Apr;96(4):514-25.
20. Haimovici R, Gragoudas ES, Duker JS, et al. Central serous chorioretinopathy associated with inhaled or intranasal corticosteroids. Ophthalmology. 1997 Oct;104(10):1653-60.
21. Abelson MB, Welch DL. An evaluation of onset and duration of action of Patanol (olopatadine hydrochloride ophthalmic solution 0.1%) compared to Claritin (loratadine 10 mg) tablets in acute allergic conjunctivitis in the conjunctival allergen challenge model. Acta Ophthalmol Scand Suppl. 2000;(230):60-3.

