|
and JAMES L. FANELLI, O.D., Contributing Editor |
A54-year-old white female presented for her yearly examination in May 2000. She had experienced a decrease in vision in her right eye over the previous three weeks. She described it as a stationary, transparent gray film. She reported no other visual changes.
She has been a patient of the practice for several years, and has a history of suspected fundus flavimaculatus and familial drusen O.U. Until this visit, her fundus appearance had remained stable. Through previous historical accounts, she disclosed that her younger sister has similar retinal findings.
Diagnostic Data
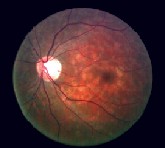
Left fundus: Note irregular areas of pigmentation. Fish flecks are most visible outside the arcades.
Best-corrected visual acuity was 20/50- O.D. and 20/20 O.S. There was no further improvement with pinhole or refraction. Pupils were equal, round and reactive to light with no afferent pupillary defect. Amsler grid testing showed metamorphopsia nasally O.D. and no defects or metamorphopsia O.S.
Slit lamp examination of the anterior segments was completely unremarkable O.U. Intraocular pressures, anterior chamber angles, optic disks and retinal vasculature were all within healthy limits in both eyes.
The maculae in both eyes were characterized by retinal pigment epithelial (RPE) windows and scattered paramacular drusen, which resembled fish flecks. Stereoscopic observation of the macula in the right eye demonstrated an elevated area of grayish appearance, about 0.25 disc diameter in size. This area was located slightly superior to the foveal avascular zone and resembled an early choroidal neovascular membrane.
Diagnosis
We suspected an occult subretinal neovascular membrane O.D.
Treatment and Follow-up
A fluorescein angiogram showed scattered drusen throughout the paramacular regions O.U.; this was consistent with the fundus appearance. In the right eye, there was an area of chorioretinal disruption superior and temporal to the foveal avascular zone. The area had one pinpoint spot of hyperfluorescence, which increased in size and intensity throughout the angiogram. This area of late leakage was highly suggestive of choroidal neovascularization.
The fish-flecked appearance of the macula and mid-periphery on angiography supported our previous diagnosis of fundus flavimaculatus (FF).
Given the unusual nature of the presentation, the angiogram was repeated one week later. It revealed the same angiographic pattern with a slight enlargement of the hyperfluorescent area.
We consulted with a retinal specialist who confirmed our diagnosis: choroidal neovascular membrane (CNVM) and fundus flavimaculatus. Further tertiary opinion by university retinologists confirmed the same.
Subsequent electroretinogram (ERG), electro-oculogram (EOG) and dark adaptation curves were all normal.
After discussion among all ophthalmic providers involved in her care, we recommended to the patient a course of focal photocoagulation of the neovascular membrane, which she elected to do. (Photodynamic therapy was not considered an option at the time of initial presentation, as the patient did not meet the criteria for PDT. Recent studies suggest a role for PDT in cases such as this.1) Submacular membrane peels were considered too risky given her relatively good visual acuity.
Discussion
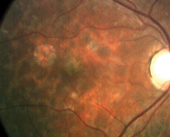
The right macula one year post-laser: The treatment area is superior temporal to the foveola inside the FAZ, adjacent to the RPE window.
When our patient initially presented approximately six years earlier, she reported a sister with some unusual retinal findings, but was unaware of the exact nature of her sisters condition. She was also unaware that she had similar findings. Given her initial, rather benign appearance of familial drusen with no macular involvement in either eye, and her absence of visual field compromise or visual disturbances, we postponed further electrodiagnostic evaluation. She remained stable until the onset of the metamorphopsia.
Differentiating the flecked retina syndromes on observation alone is a challenge. This group of inherited diseasesincluding albipunctate dystrophies, familial drusen, fleck retina of Kandori and Stargardts disease (fundus flavimaculatus)often require ancillary testing to help confirm the diagnosis. Along with a dilated fundus exam and fluorescein angiography, testing includes ERG, EOG, dark adaptation curves, color vision and visual fields.
Fundus flavimaculatus and Stargardts disease are often viewed as different variations of the same disease process. However, the two disease processes may have significant differences that, in time, may lead to segregation of the two variations.
In managing patients with FF and Stargardts disease, be aware that the changes in RPE morphology include heterogeneity in cell size, apical displacement of pigment granules and the abnormal accumulation of intracytoplasmic material.
Familial drusen is also a hereditary flecked retina syndrome with fundus findings of bilaterally symmetric, deep, yellow-white depositions that may develop disciform detachments with or without subretinal neovascularization. The histopathology of familial drusen mimics that of AMD as globular hyaline bodies from metabolic waste products from RPE cells become deposited into Bruchs membrane.
While choroidal neovascularization occurs more frequently in familial drusen, it has been infrequently reported in cases of FF.
A study from the Vitreoretinal Foundation looked at shared characteristics of Stargardts macular dystrophy and fundus flavimaculatus.2 Researchers observed 52 patients with Stargardts macular dystrophy and 48 patients with FF for a period ranging from one to 22 years. They found choroidal neovascular membranes in a total of four eyes in three patients who otherwise had no obvious etiologic factors for the development of CNVM. Although choroidal neovascular membranes are usually not associated with FF, this finding suggests that the disease process may accelerate the usual aging changes of the central retina-RPE complex, predisposing these patients to such complications at an early age.
So, changes associated with FF may increase the risk for development of choroidal neovascular membranes. Take care when evaluating these patients, as the potential for choroidal neovascular membranesalthough raredoes exist. Routine evaluations, patient education and quick action by eye-care practitioners are important parts of the ongoing care.
When last seen in October 2002, this patients best-corrected visual acuity was still 20/40- O.D., with positive Amsler findings. Her acuity, Amsler grid tests and FAs have remained stable since her CNVM resolved (as determined by angiogram) in early 2001.
Dr. Comerford is in practice in Radford, Va. Dr. Fanelli is in private practice in Wilmington, N.C., and is author of Review of Optometrys Glaucoma Grand Rounds column.
1. Valmaggia C, Niederberger H, Helbig H. Photodynamic therapy for choroidal neovascularization in fundus flavimaculatus. Retina 2002 Feb;22(1):111-3.
2. Armstrong JD, Meyer D, Xu S, Elfervig JL. Long-term follow-up of Stargardts disease and fundus flavimaculatus. Ophthalmology 1998 Mar;105(3):448-58.

