When patients complain about the lingering visual disturbances that follow dilation, their eye doctors don’t have much to offer beyond reassurance that the procedure allowed them to have the most thorough eye exam possible. Early next year, however, optometrists and ophthalmologists will be able to use a topical medication that cuts the recovery time down significantly, according to Viatris and Ocuphire Pharma, the drug’s developers.
This morning, the companies announced the FDA approval of Ryzumvi (phentolamine ophthalmic solution 0.75%) for the treatment of pharmacologically induced mydriasis produced by adrenergic agonists (e.g., phenylephrine) or parasympatholytic (e.g., tropicamide) agents, and said they expect it to be available in the first half of 2024.
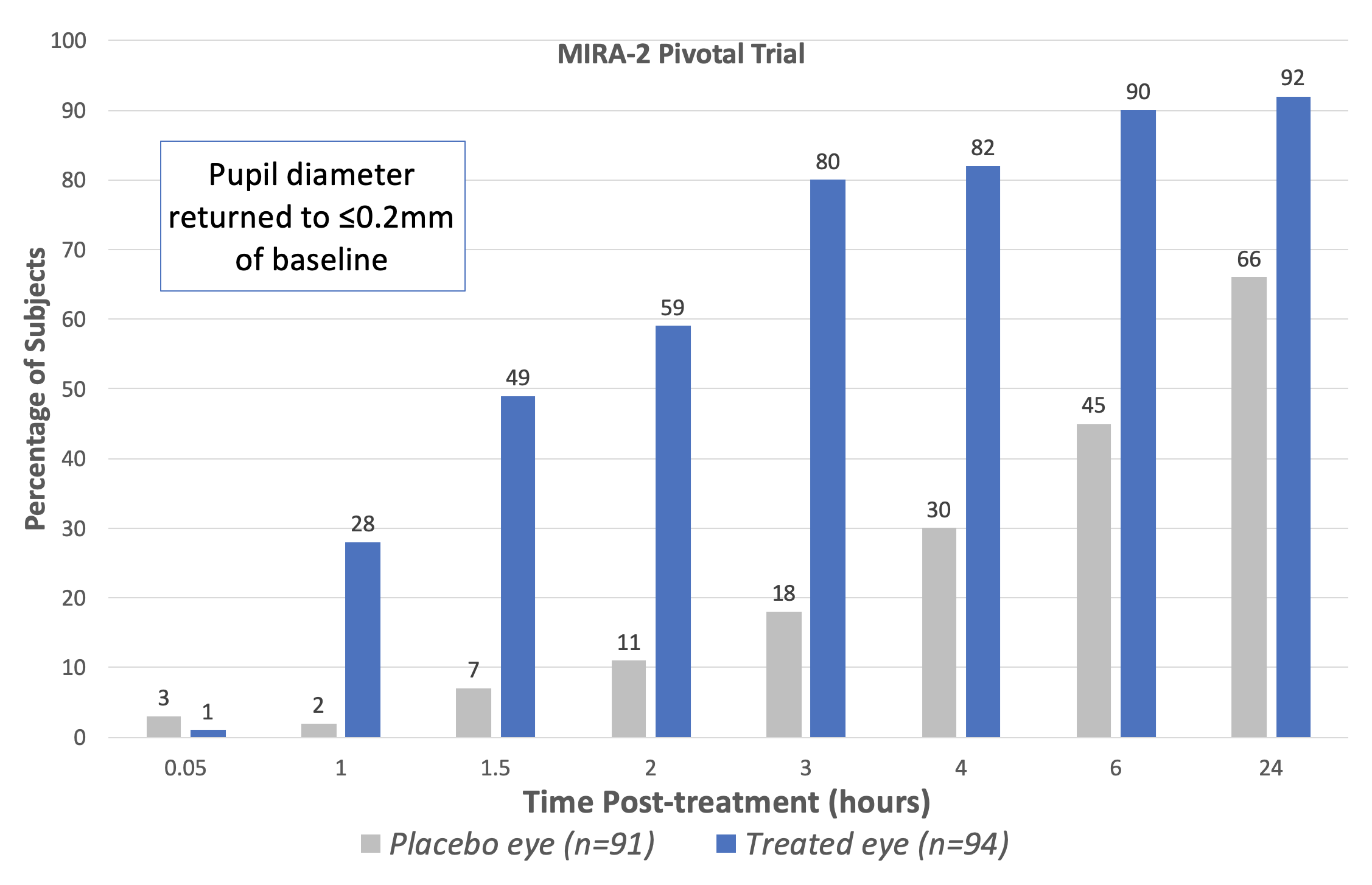 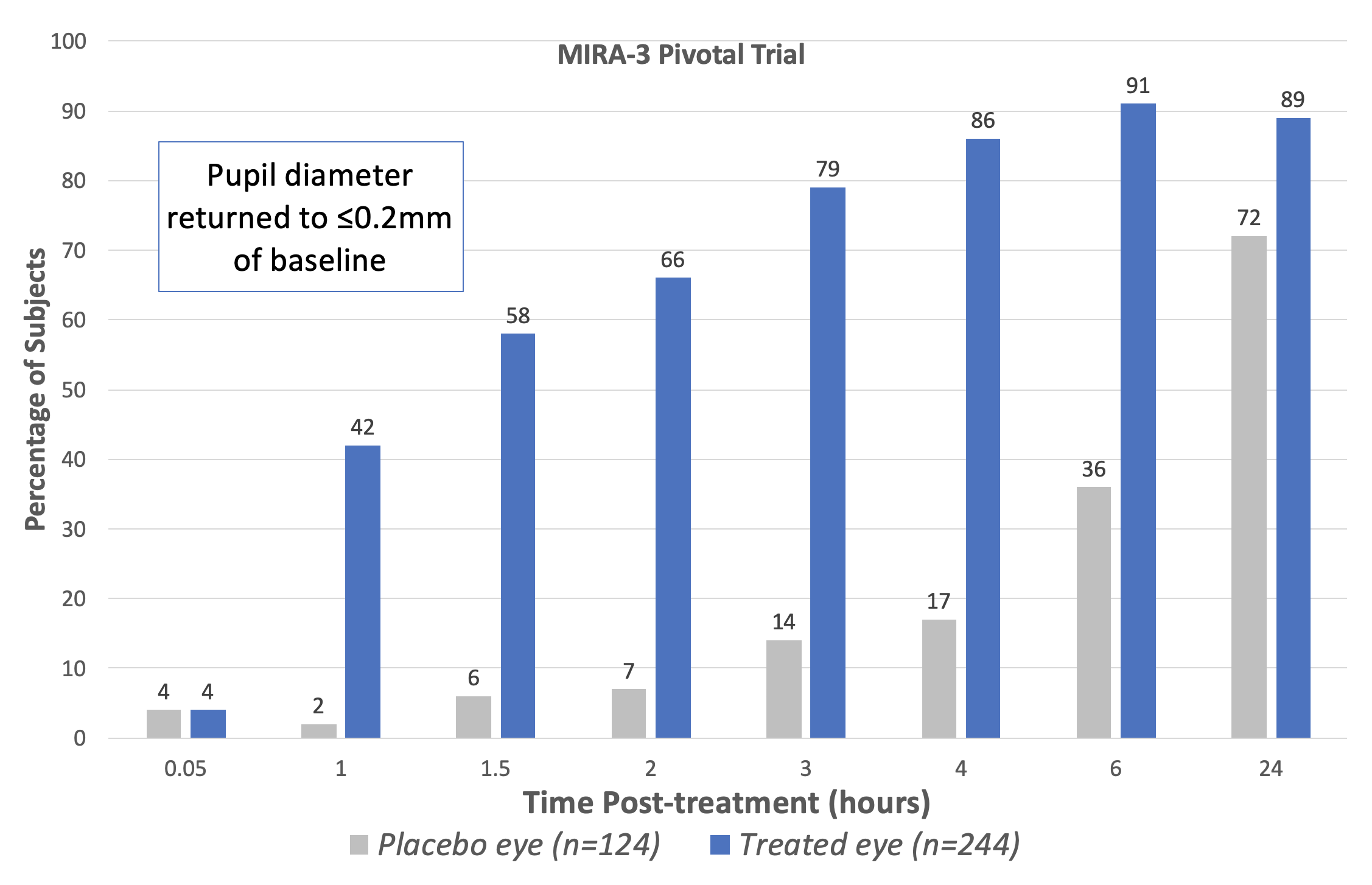 |
| The Phase III pivotal trials (MIRA-2 and MIRA-3) showed that treatment with Ryzumvi restored pupils to pre-dilated status faster than untreated subjects. (Adapted from Prescribing Information statement; data for fellow eye treatment omitted.) Click image to enlarge. |
In two Phase III pivotal trials (MIRA-2 and MIRA-3), a total of 553 subjects aged 12 to 80 years with drug-induced mydriasis received two drops of Ryzumvi in the study eye (and one drop in the fellow eye) one hour following instillation of the dilating agent. The percentage of study eyes returning to ≤0.2mm from baseline pupil diameter was statistically significantly greater at all time points measured from 60 minutes through 24 hours vs. placebo (vehicle), a press release explains. Efficacy was similar for all age ranges, including pediatric subjects aged 3 to 17 years.
For adults and pediatric patients age 12 years and older, doctors are instructed to instill one to two drops in each dilated eye following the completion of the ophthalmic examination or procedure. For children age three to 11, the dose should be just one drop. The drug should not be used in patients currently experiencing iritis.
The most common ocular adverse reactions reported in >5% of subjects were instillation site discomfort including pain, stinging and burning (16%) and conjunctival hyperemia (12%). The only non-ocular adverse reaction reported in >5% of subjects was dysgeusia (6%).
More information is available at www.ryzumvi.com.

