The search for more potent, efficacious and target-selective drugs with minimal side effects remains an ongoing quest. It is a perpetual battle in the fight against diseases and, in the case of infections, the constant struggle against the development of resistant microorganisms.
We aim to target different mechanisms of the disease and microorganisms to create a drug that is more efficacious, potent and selective with minimal adverse or side effects. We can accomplish this by synthesizing new drugs, modifying the molecule or the preparation of existing drugs, as well as by improving the kinetics, spectrum, efficacy, potency and adverse effect profile. Fortunately, investigators are making progress.
Here is a preview of some of the drugs in development in the ophthalmic pipeline.
Glaucoma
In glaucoma treatment, a new class of compounds—the Rho-kinase inhibitors (ROCK)—are at the forefront of promising drugs under study.
The Rho-kinase pathway is an integral part of various important cellular functions, such as contraction of vascular smooth muscle cells, organization of the actin cytoskeleton, cell adhesion and motility, and gene expression.1

ROCK inhibitors differ from prostaglandin analogs because their mechanism of action targets outflow through the trabecular meshwork rather than the prostaglandin’s uveoscleral outflow.
ROCK inhibitors differ from the prostaglandin analogs—currently the first-line drugs in glaucoma therapy—in that their mechanism of action targets the conventional outflow facility through the trabecular meshwork in the anterior segment, rather than the uveoscleral outflow in the posterior segment. One study reported that treatment with a ROCK inhibitor, Y-27632, affected human trabecular meshwork and Schlemm’s canal cells to produce reversible changes in cell shape, focal adhesions and decreases in stress of the actin fibers. This resulted in an increase in permeability of the Schlemm cells’ monolayer by 80%.2 Also, Y-27632 increased aqueous outflow facility by 40% to 80% in enucleated porcine eyes. This effect was due to the increase in extracellular spaces of the juxtacanalicular tissue.2
ROCK inhibitors have been shown to decrease intraocular pressure by 25% to 32% and have a duration of action of 10 to 12 hours, which suggests that the drug will be administered twice a day vs. once daily like the prostaglandin analogs. Nevertheless, this type of drug may be a great addition to the medication regimen for patients on a prostaglandin who fail to achieve the target pressure required to prevent progression of the disease. Also, it may be indicated for patients who cannot tolerate the prostaglandin analogs.
Several ROCK inhibitors and other glaucoma drugs are now in development:
• ATS907. Atheos Inc. is running a Phase IIa clinical trial of ATS907, a ROCK-selective inhibitor for the reduction of intraocular pressure in ocular hypertensive and primary open angle patients. (ATS907 is one of a series of ROCK inhibitor compounds licensed from Asahi Kasei Pharma.) Preclinical data suggest that, in addition to its anti-inflammatory ability, ATS907 may have a neuroprotective effect and increase perfusion of the optic nerve.3
• ATS8535. Researchers reported on the efficacy of ATS8535 on IOP decrease in monkeys and in rabbits. Following a single dose of ATS8535 0.2% in monkeys, IOP decreased by 5mm Hg two to four hours post-dosing (from a mean baseline of 21.5-22.5mm Hg), and lasted for 10 to 12 hours. Similarly, one drop of ATS8535 0.08% produced a decrease of 7mm Hg in rabbits (from a mean baseline of 21.5-22.5mm Hg).4 Atheos has classified this drug as a candidate for development.
• AR-12286. This ROCK inhibitor, developed by Aerie Pharmaceuticals, is advancing to Phase III clinical trials. It demonstrated good efficacy, safety and tolerability in early trials.5 AR-12286 is also being developed in a fixed-dose combination with travoprost, which is in Phase III clinical trials; it demonstrated good additive effect with travoprost without producing the excessive hyperemia.6
• AR-13324. This lowers IOP by a dual mechanism—by increasing the outflow through the trabecular meshwork and by inhibiting the norepinephrine transporter to decrease aqueous production. In a 10-day rabbit study, a once-daily drop of AR-13324 0.04% reduced IOP by up to 7.1mm Hg at four hours post-dosing (from a mean baseline of 24.8-28.0mm Hg).7 A study in normotensive monkeys reported that a single-dose administration of AR-13324 0.04% increased outflow facility by 53% after six hours, while the aqueous inflow decreased by 23%, and IOP decreased by 25%.8
• AMA0076. This potent ROCK inhibitor, being developed by Ama-kem NV, is intended to permit high-dose topical administration with minimal systemic side effects.9 Investigators recently presented the results of their study on the effect of benzalkonium chloride (BAK) on the pressure-lowering ability of AMA0076 in rabbits. They compared AMA0076 0.1%, 0.3% and 0.5% with and without 0.015% BAK. After the administration of one drop of AMA0076 with and without BAK unilaterally, IOP declined by 21%, 28% and 37% for 0.1%, 0.3% and 0.5% respectively without BAK, while IOP decreased by 38%, 45% and 53% with BAK.9
• BOL-303259-X. This is not a ROCK inhibitor, but a novel nitric oxide-donating prostaglandin F2-alpha analog developed by NicOx S.A. and now investigated by Bausch + Lomb. Nitric oxide has vasodilatory properties and plays a role in inflammatory and immune responses, reproductive functions, bronchodilation and other processes.
Top-line results from a Phase IIb study that compared once-nightly BOL-303259-X vs. once-nightly latanoprost 0.005% showed notable reduction in mean diurnal IOP at one month.10 Two of the four doses tested showed greater IOP reduction than that achieved with latanoprost. The most efficacious dose of BOL-303259-X also showed consistently better IOP control over 24 hours, as well as a statistically significant greater percentage of responders (patients who achieved an IOP of 18mm Hg or less) compared to latanoprost. The responder rate was 68.7% for the most efficacious dose of BOL-303259-X compared to 47.5% for latanoprost.10 The drug’s safety and adverse events (i.e., hyperemia) were comparable to latanoprost. Bausch + Lomb anticipates a Phase III trial in the future.
Anti-Infectives
• NVC-422. This aganocide compound, developed by NovaBay, is beginning a Phase IIb clinical trial to treat adenoviral conjunctivitis.
Aganocides belong to a novel class of compounds that mimic the body’s natural defense against infection. The aganocides have demonstrated a greater in vitro therapeutic index than existing topical antiseptics. Aganocides also possess a reduced likelihood to develop resistance to bacteria or viruses.
NVC-422 is the first synthetic N-chlorinated taurine molecule to maintain antimicrobial activity and improved stability over the body’s naturally occurring N-chlorinated taurine. It exhibits a broad spectrum of activity against bacteria, viruses, yeasts and fungi. It is also effective against multi-drug resistant bacteria, such as methicillin-resistant Strep. aureus (MRSA) and vancomycin-resistant Enterococcus (VRE).11 NVC-422 is fast acting; it can eradicate bacteria in minutes, even at low doses.
Different antimicrobial topical drops are being developed to target adenoviral conjunctivitis. Photo: Ron Melton, O.D., and Randall Thomas, O.D.
NovaBay Pharmaceuticals is investigating a topical ophthalmic formulation of NVC-422 for viral conjunctivitis.12 The drug is also being developed for dermatology, urology and hospital-based infections.
• FST-100. This is another antibiotic for the treatment of adenoviral conjunctivitis. Developed by Foresight Biotherapeutics, FST-100 is a combination suspension that targets microbial eradication (using povidone-iodine) while decreasing the inflammation associated with ocular infections (using dexamethasone).13 Povidone-iodine is a broad-spectrum antiseptic with antimicrobial activity; it is widely used as a surgical scrub and as a prophylactic for neonatal conjunctivitis. Its mechanism of action is iodination of lipids and oxidation of cytoplasmic and membrane compounds, and it has minimal development of resistance.13 While the dexamethasone is not synergistic with the antiviral effect of the povidone-iodine, it minimizes the irritating and associated inflammatory effect of the povidone-iodine solution. A Phase II trial is scheduled to begin in October.
Anti-Inflammatory Agents
Several novel anti-inflammatory agents have been developed to deliver the power of corticosteroids, but without the adverse effects.
• Luveniq. Lux Biosciences has completed Phase III trials of Luveniq (voclosporin, formerly LX211) an oral capsule for the treatment of non-infectious uveitis. Voclosporin, a novel immunomodulatory drug that inhibits the calcineurin enzyme, was originally developed to prevent organ graft rejection and to treat autoimmune diseases. The chemical structure of voclosporin is similar to that of cyclosporine A, but with a difference in one amino acid, leading to superior calcineurin inhibition and less variability in plasma concentration.14
Lux Biosciences anticipates that data from the Phase III trials will be available in early 2013. (The company had previously submitted a New Drug Application for Luveniq in 2010, but the FDA requested additional clinical data. This trial aims to satisfy the FDA’s request.)
• ESBA105. This is a single-chain antibody fragment that targets tumor necrosis factor-alpha (TNF-alpha), a major mediator of inflammation.15 Selective inhibition of TNF-alpha has the potential of modulating the inflammatory and immune response. ESBA105 will be indicated in non-infective inflammatory diseases of the eye.
Preclinical studies have demonstrated that topically administered ESBA105 attains therapeutic levels in both the anterior and posterior segments of the eye, and may have potential for the treatment of ocular diseases mediated by TNF-alpha.16
Here
are just a few of the many promising ophthalmic medicines that are
worth noting, although they’re still in the early stages of development: The drop is
administered with a novel delivery system (the VersiDoser, developed by
Mystic Pharmaceuticals) that employs unit-dose, micro-pump “blisters”
that are inserted into a small handheld pump sprayer to deliver a
preservative-free uniform spray dose to the ocular surface.
The
FDA has given MC-1101 official “Fast Track” status. A Phase II trial is
in the works that will evaluate both the drug (dosed t.i.d.) as well as
the vehicle.
• EBI-005.
Eleven Biotherapeutics is developing EBI-005, an interleukin-1 receptor
inhibitor for the treatment of dry eye syndrome.
In
an inflammatory reaction, an abundance of cytokines, interleukin-1 and
tumor necrosis factor are released, which stimulates the attached
T-helper cell to secrete another cytokine, interleukin-2. The
interleukin-2 stimulates the helper cell to multiply and develop
specific antibodies to ward off the foreign objects. But
by inhibiting the interleukin-1 receptor, EBI-005 halts the cascade and
cuts short the inflammatory response. It was found to be more active
than topical cyclosporine in a mouse model of dry eye syndrome.27 •
SIRT1460. The SIRT1 protein is an enzyme (histone deacteylase) that has
the ability to suppress the expression of a variety of genes and thus
influence a wide variety of biological processes.28
Activating
the SIRT1 protein together with a coenzyme (NAD) causes a deacetylation
of the histone protein, which in turn silences the expression of the
gene, which decreases the function of the proteins that promote the
aging process. Thus, activation of the SIRT1 protein may play a
significant role in metabolic, cardiovascular, neurodegenerative
diseases and in inflammation. In short, this process is believed to
extend lifespan. SIRT1
activators may have potential as a novel class of compounds for the
treatment of dry eye disease. For instance, investigators tested
SIRT1460, dosed b.i.d., in a dry eye study in mice and significantly
decreased corneal fluorescein staining (by 26% in the low-dose group and
by 49% in the high-dose group).29
A Phase IIa study of the drug, conducted by developer ESBATech, is directed at patients with acute anterior uveitis. The company has since been acquired by Alcon, which is conducting a Phase II trial of ESBA105 for severe dry eye. Topical application of ESBA105 also reduced the formation of choroidal neovascularization, indicating its use in the prevention and treatment of age-related macular degeneration.17
Further Up the Pipeline
•
MC-1101. This proprietary topical drop, developed by MacuClear, aims to
stop dry AMD from progressing to wet AMD. A Phase Ib proof-of-concept
trial found that topical instillation of MC-1101 was not only safe and
well tolerated, but it also reached the macula, relaxed the epithelial
lining of the vasculature, dilated choroidal blood vessels by
stimulating nitric oxide production, and increased choroidal blood
circulation.26
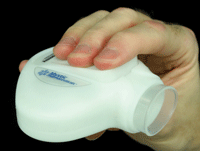
MC-1101 will be administered with a novel pump sprayer, which is filled with a cartridge of unit-dose blisters.
• Mapracorat. Mapracorat (ZK 245186/BOL-303242-X, developed by Bausch + Lomb) is a new type of anti-inflammatory with a non-steroidal chemical structure for the treatment of anterior segment conditions. It is a selective glucocorticoid receptor agonist (SEGRA)—it is designed with similar anti-inflammatory and immunosuppressive effects as the glucocorticoids but with a decreased potential of the steroid side effects.18
Currently, a Phase II study is evaluating its effectiveness in preventing the signs and symptoms of allergic conjunctivitis. In addition, a Phase III study is underway for the treatment of ocular inflammation after cataract surgery.
• Nepafenac 0.3%. Alcon Laboratories has completed a Phase III clinical trial of a new 0.3% formulation of its NSAID Nevanac (currently approved as nepafenac 0.1%) for the treatment and prevention of pain and inflammation following cataract surgery.19 Although this is the same active ingredient as in Nevanac, the formulation is being changed to improve the pharmacokinetics and/or to modify or change the preservative. No study results have been posted yet.
• BromSite. InSite Vision announced favorable results from Phase I/II clinical trials of BromSite (or ISV-303, 0.075% bromfenac in its patented DuraSite polymer vehicle), a topical anti-inflammatory agent for the reduction of pain and inflammation after cataract surgery.20 The Phase II pharmacokinetic study compared BromSite vs. bromfenac 0.09% alone, and found BromSite dosed b.i.d. had more than twice the mean concentration in the aqueous humor compared to bromfenac b.i.d. InSite Vision is now recruiting subjects for a Phase III trial.
• DexaSite. The active ingredient in InSite Vision’s DexaSite (or ISV-305) is low-dose (0.1%) dexamethasone formulated in its DuraSite vehicle. DexaSite is intended to decrease the signs and symptoms of non-infectious blepharitis. So far, results show that it is a safe and efficacious therapy for blepharoconjunctivitis.21 The start date for its Phase III trial is March 2013, with completion expected in January 2014.
• AzaSite Plus. Another entry from InSite Vision, AzaSite Plus (or ISV-502) is topical azithromycin 1% (like regular AzaSite) with the addition of dexamethasone 0.1%. Besides their antimicrobial activity, the macrolide antibiotics (including azithromycin) also have been shown to possess anti-inflammatory properties.22 The addition of dexamethasone to this formulation should provide a genuine anti-inflammatory effect.23 InSite Vision currently is recruiting participants for a new Phase III study for the treatment of non-bacterial blepharitis.
Dry Eye Drugs
In the area of dry eye treatment, new pharmaceutical development is targeting aspects of the inflammatory cascade.
• CF101. Adenosine is a neurotransmitter with a very short half-life that not only acts peripherally, but also has a role in the central nervous system. Adenosine has been shown to inhibit leukotriene B4 (LTB4), which is part of the arachidonic acid cascade for the synthesis of prostaglandins and leukotrienes.
An adenosine A3 receptor agonist, CF101, is a small molecule drug that has shown efficacy and an excellent safety profile in Phase II trials.24 Dosed orally once daily, the drug is now in a multi-centered Phase III clinical trial of patients with moderate to severe dry eye. It is concurrently being developed for uveitis and glaucoma. The dry eye and uveitis indications take advantage of the anti-inflammatory effects of the drug. The rationale for conducting the glaucoma study (now in Phase II) is due to an unexpected IOP decrease of 1.1mm Hg in its Phase II dry eye trial.24
• Lifitegrast. Previously known as SAR 1118, developed by SARcode Bioscience, lifitegrast is a potent and selective small molecule drug being investigated for the treatment of dry eye and ocular allergy. It inhibits T-cell inflammation by blocking the binding of two key cellular surface proteins that mediate the chronic inflammatory cascade.
In a Phase II trial of 230 dry eye patients, lifitegrast was well tolerated with mild ocular adverse events, and demonstrated significant improvements in tear production and symptoms within two weeks.25 A Phase III trial for dry eye is currently recruiting subjects. The Phase II study for allergic conjunctivitis has been completed, but no results have yet been announced.
There are a number of other new and emerging ophthalmic drugs in the pipeline—too many to include in this article.
Sometimes we get frustrated with the many conditions we can’t treat or have difficulty treating. But if you stop to consider the various drugs currently available and the number of new meds coming our way, this really is a good time to be in eye care.
Dr. Chang is a professor at Southern College of Optometry and teaches courses and laboratories in the areas of pharmacology, ocular physiology, systemic health and contact lenses.
1. Shimokawa H, Takeshita A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol. 2005 Sep;25(9):1767-75.
2. Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001 Apr;42(5):1029-37.
3. Altheos website. Altheos Announces Initiation of Phase 2a Clinical Trial for its Lead Glaucoma Drug Candidate, ATS907. Available at:
http://altheos.net/9-February-2012-Phase-2a-Clinical-Trial.html. Accessed August 23, 2012.
4. Wirostko BM, Umeno H, Hsu H, Kengatharan M. Safety and efficacy of a novel topical Rho kinase inhibitor ATS8535 in vivo. #5079/A220. Poster presented at Association for Research in Vision and Ophthalmology meeting, May 9, 2012; Ft. Lauderdale, Fla.
5. Williams RD, Novack GD, van Haarlem T, Kopczynski C. AR-12286 Phase 2A Study Group. Ocular hypotensive effect of the Rho kinase inhibitor AR-12286 in patients with glaucoma and ocular hypertension. Am J Ophthalmol. 2011 Nov;152(5):834-41.
6. Kopczynski CC. Rho kinase inhibitors for glaucoma. Presentation at 4th Ocular Diseases & Drug Discovery meeting, Feb. 27, 2012; Las Vegas.
7. Kopczynski C, Lin C-W, deLong M, et al. IOP lowering efficacy and tolerability of AR-13324, a dual mechanism kinase inhibitor for the treatment of glaucoma. #5080/A221. Poster presented at Association for Research in Vision and Ophthalmology meeting, May 9, 2012; Ft. Lauderdale, Fla.
8. Wang RF, Serle J, Kopczynski C. Effect of 0.04% AR-13324 on aqueous humor dynamics in normotensive monkey eyes. #1994/D838. Poster presented at Association for Research in Vision and Ophthalmology meeting, May 7, 2012; Ft. Lauderdale, Fla.
9. Hollanders KP, Sijnave D, Van Bergen, et al. The effect of benzalkonium chloride on the intraocular pressure lowering efficacy of a local ROCK-inhibitor (AMA0076). #1974/D818. Poster presented at Association for Research in Vision and Ophthalmology meeting, May 7, 2012; Ft. Lauderdale, Fla.
10. Bausch + Lomb website. NicOx, Bausch + Lomb glaucoma candidate BOL 303259 X meets primary endpoint in Phase 2b study. March 13, 2012. Available at:
www.bausch.com/en/Our-Company/Recent-News/2012-Archive/NicOx. Accessed August 28, 2012.
11. Wang L, Belisle B, Bassiri M, et al. Chemical characterization and biological properties of NVC-422, a novel, stable N-chlorotaurine analog. Antimicrob Agents Chemother. 2011 Jun;55(6):2688-92.
12. Yoon J, Jekle A, Najafi R, et al. Virucidal mechanism of action of NVC-422, a novel antimicrobial drug for the treatment of adenoviral conjunctivitis. Antiviral Res. 2011 Dec;92(3):470-8. Epub 2011 Oct 15.
13. Clement C, Capriotti JA, Kumar M, et al. Clinical and antiviral efficacy of an ophthalmic formulation of dexamethasone povidone-iodine in a rabbit model of adenoviral keratoconjunctivitis. Invest Ophthalmol Vis Sci. 2011 Jan 21;52(1):339-44.
14. Roesel M, Tappeiner C, Heiligenhaus A, Heinz C. Oral voclosporin: novel calcineurin inhibitor for treatment of noninfectious uveitis. Clin Ophthalmol. 2011;5:1309-13.
15. Furrer E, Berdugo M, Stella C, et al. Pharmacokinetics and posterior segment biodistribution of ESBA105, an anti-TNF-alpha single-chain antibody, upon topical administration to the rabbit eye. Invest Ophthalmol Vis Sci. 2009 Feb;50(2):771-8.
16. Ottiger M, Thiel MA, Feige U, et al. Efficient intraocular penetration of topical anti-TNF-alpha single-chain antibody (ESBA105) to anterior and posterior segment without penetration enhancer. Invest Ophthalmol Vis Sci. 2009 Feb;50(2):779-86.
17. Lichtlen P, Lam TT, Nork TM, et al. Relative contribution of VEGF and TNF-alpha in the cynomolgus laser-induced CNV model: comparing the efficacy of bevacizumab, adalimumab, and ESBA105. Invest Ophthalmol Vis Sci. 2010 Sep;51(9):4738-45.
18. Ehrchen J, Steinmüller L, Barczyk K, et al. Glucocorticoids induce differentiation of a specifically activated, anti-inflammatory subtype of human monocytes. Blood. 2007 Feb 1;109(3):1265-74.
19. ClinicalTrials.gov website. Confirmatory Study Nepafenac 0.3%. Last Updated on July 17, 2012. Available at:
http://clinicaltrials.gov/ct2/show/NCT01109173. Accessed August 30, 2012.
20. InSite Vision website. InSite Vision Initiates the First Phase 3 Clinical Study of BromSite for the Reduction of Pain and Inflammation after Cataract Surgery. July 31, 2012. Available at:
http://phx.corporate-ir.net/phoenix.zhtml?c=86061&p=irol-newsArticle&ID=1720524. Accessed August 30, 2012.
21. Hosseini K, Hutcheson J, Bowmam LM. Clinical response of dexamethasone 0.1% formulated in DuraSite on blepharoconjunctivitis patients. #4247/D991. Poster presented at Association for Research in Vision and Ophthalmology meeting, May 4, 2011; Ft. Lauderdale, Fla.
22. Ianaro A, Ialenti A, Maffia P, et al. Anti-inflammatory activity of macrolide antibiotics. J Pharmacol Exp Ther. 2000 Jan;292(1):156-63.
23. Si EC, Hutcheson J, Hosseini K. Antimicrobial efficacy of azithromycin/dexamethasone combination in blepharoconjunctivitis patients, a Phase 3 study. #2888/D1112. Poster presented at Association for Research in Vision and Ophthalmology meeting, May 4, 2010; Ft. Lauderdale, Fla.
24. Avni I, Garzozi HJ, Barequet IS, et al. Treatment of dry eye syndrome with orally administered CF101: data from a phase 2 clinical trial. Ophthalmology. 2010 Jul;117(7):1287-93.
25. Semba CP, Torkildsen GL, Lonsdale JD, et al. A phase 2 randomized, double-masked, placebo-controlled study of a novel integrin antagonist (SAR 1118) for the treatment of dry eye. Am J Ophthalmol. 2012 Jun;153(6):1050-60.
26. Ralston PG Jr., Sloan D, Waters-Honcu D, et al. A pilot, open-label study of the safety of MC-1101 in both normal volunteers and patients with early nonexudative age-related macular degeneration. #913/A196. Paper presented at Association for Research in Vision and Ophthalmology meeting, May 2, 2010; Ft. Lauderdale, Fla.
27. Furfine ES, Hou J, Collins K, et al. EBI-005: an interleukin-1 receptor inhibitor designed for the treatment of dry eye syndrome. #2340/A357. Poster presented at Association for Research in Vision and Ophthalmology meeting, May 7, 2012; Ft. Lauderdale, Fla.
28. Herranz D, Muñoz-Martin M, Cañamero M, et al. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun. 2010 Apr 12;1:3.
29. Krauss AH, Amparo F, Okanobo A, et al. Improvement of corneal staining by a Sirt1 activator in a mouse model of dry eye. #2343/A360. Poster presented at Association for Research in Vision and Ophthalmology meeting, May 7, 2012; Ft. Lauderdale, Fla.

