 |
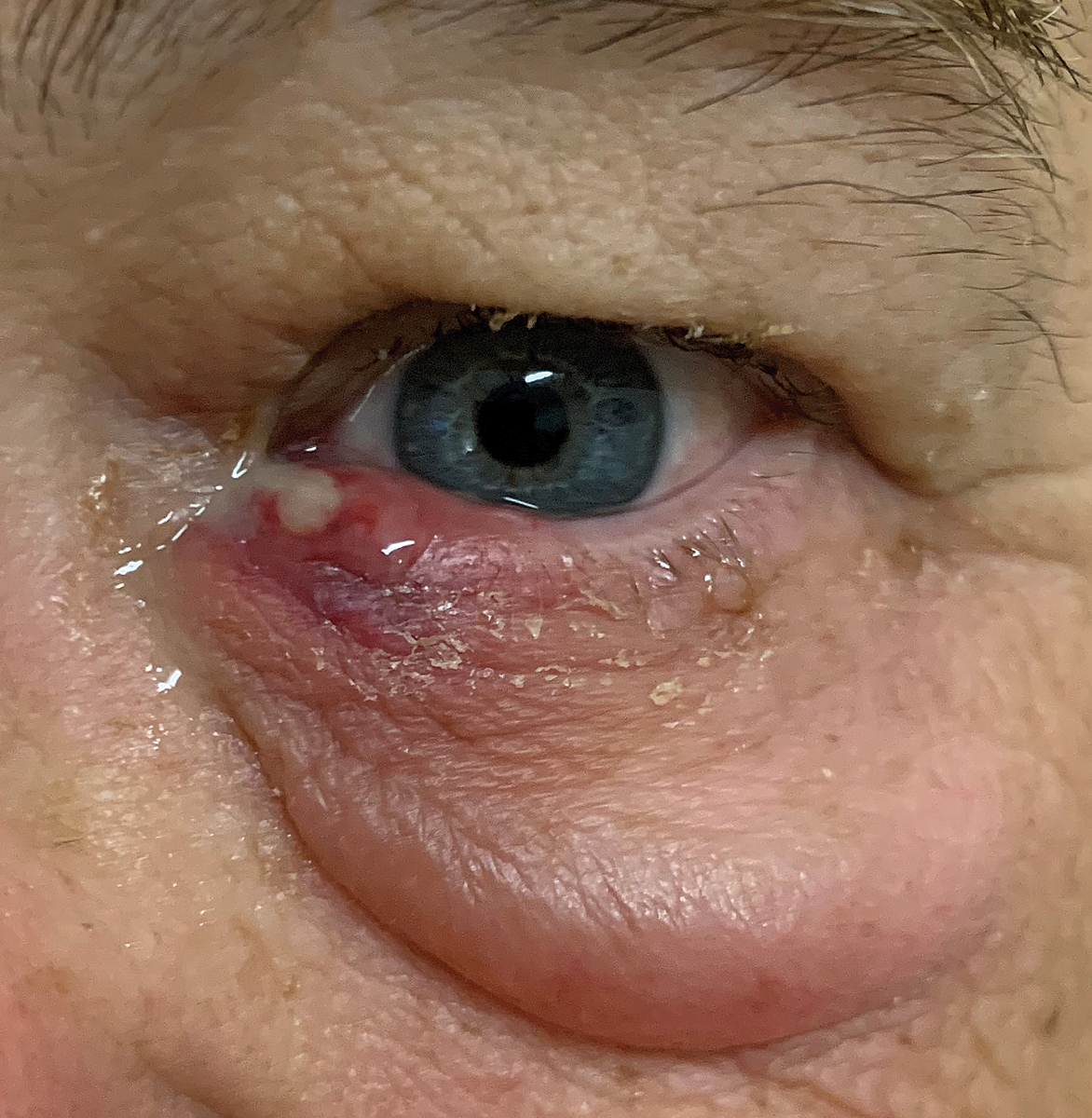
A 62-year-old man presented emergently with a painful, swollen left lower eyelid of two days duration. His eyelid was acutely painful in the nasal region, and he had a mucopurulent discharge emanating from his lower puncta. His vision was unchanged, and he had no other complaints. His medical history was significant for thyroid dysfunction and elevated cholesterol, for which he was using medications. He was concerned that a family member living in his house currently had a methicillin-resistant Staphylococcus aureus (MRSA) infection.
He had a profound amount of lower eyelid edema and focal swelling in the canthal and puncta area. When palpated, he reported significant discomfort, and there was expression of mucopurulence. Clearly, the diagnosis was acute (presumptive) bacterial dacryocystitis.
 |
|
Symptoms of dacryocystitis include a painful, red and swollen tear sac that may produce a pus-like material that seeps out of the punctum when pressure is applied as seen in the patient presented. Click image to enlarge. |
Background
Acute dacryocystitis, also sometimes referred to as lacrimal sac mucocele, is an infection of the lacrimal sac.1-7 It occurs from trauma, lacrimal system obstruction or bacterial infection.1-8 Dacryocystitis may also result from an extension of infective and inflammatory processes occurring within the nose or paranasal sinuses.3,4 In some instances, the infection (initially confined to the lacrimal sac) can extend to the soft tissues, causing preseptal cellulitis, or invade the orbital contents, resulting in orbital cellulitis.1-7 The condition is typically unilateral.
Acute dacryocystitis often presents with symptoms of severe pain of the inner canthus in the area of the lacrimal sac just under the medial canthal ligament. Local redness, swelling, epiphora, secondary conjunctivitis, mucoid discharge in the morning and an enlarged, lacrimal sac that is tender to the touch are typical features. Highly diagnostic is mucopurulent discharge regurgitating from the puncta when palpated.1-8 A firm round nodule is often palpable in the setting of adjacent orbital or preseptal cellulitis.1-7 Patients with acute disease are rarely febrile.
The nasolacrimal ducts consist of the upper and the lower lacrimal canaliculus, the lacrimal sac and the nasolacrimal duct, draining tears into the lower meatus of the nose.9-18 The puncta define the outermost boundary and beginning of the nasolacrimal apparatus. Each punctum respectively leads into the superior and inferior canaliculus, which extends 2mm vertically to the ampulla. From there, the canaliculus makes its turn medially toward the nasolacrimal sac.11,12 In 90% of the population, the superior and inferior canaliculi come together to form a common canaliculus that drains into the lacrimal sac. In the other 10% of the population, the superior and inferior canaliculi connect directly into the lacrimal sac.11
The epithelium lining of the lacrimal sac and the nasolacrimal duct is covered by microvilli.9 Antimicrobial peptides, IgA and immunocompetent cells (lymphocytes and macrophages) provide an antimicrobial defense.9 Under normal circumstances, the embedded blood vessels of the system maintain vegetative control deep within the system known as the cavernous body.9 Malfunctions in the cavernous body and/or in its innervations may lead to disturbances in the tear flow cycle, creating ocular congestion or total occlusion of the lacrimal passage.
Any descending infection from the eye or ascending infection from the nose or sinuses can initiate swelling of the mucous membrane and remodeling of the tissues, creating malfunctions in the cavernous body with reactive immunomodulation and occlusion of the lacrimal passage. Alterations in the ductal epithelium and the lamina propria encompassing the lacrimal sac and nasolacrimal duct can permit microbial growth.
The most common gram-positive infective organisms include Streptococcus pneumonia, Staph. aureus and Staph. epidermidis, while Haemophilus influenza, Pseudomonas aeruginosa, Serratia marcescens and Klebsiella pneumoniae are the leading the gram-negative bacteria.1,2,19 Mononucleosis and coliform bacteria are rare but documented sources of dacryocystitis.5,20
In adults, gram-negative bacteria are more common, while in children, gram-positive isolates are still the major infection pathogen. Virulent organisms are more common in acute dacryocystitis than chronic dacryocystitis.21 MRSA infections also occur more commonly in acute dacryocystitis.22
Passing the BatonAfter 18+ years of writing Therapeutic Review, I find it time to sign off and make way for Review of Optometry to give another an opportunity to provide a fresh perspective. I have enjoyed providing readers with clinically relevant content and having the opportunity to work with numerous editors over the years. I am indebted to the guidance, vision and friendship of Editor-in-Chief Jack Persico, as he was my biggest supporter all these years. I will remain with Review in an editorial capacity and will write manuscripts from time to time.
I am pleased to introduce readers to my more-than-worthy successor, Jessica Steen, OD. I have had the pleasure of working with Dr. Steen for several years. We began working together when she did her residency with me at Nova Southeastern University and continued when she joined me on faculty. She always impressed me with her intellect, insight and academic and clinical ability. Over the years, I have seen her professionally develop exponentially and rapidly learn things that took me years longer to achieve. She has blossomed into a leader in the profession and represents our future. It is truly gratifying when the student surpasses the teacher. I wish her the best in this endeavor. Cheers, Joe |
Treatment
Gentle digital massage (to express the contents of the sac) can be attempted for lesions discovered early.23 Unfortunately, this therapy tends to be ineffective, with less than 25% of lesions resolving spontaneously or with hot compresses alone.20
Acute dacryocystitis in adults is best managed with warm compresses, topical antibiotic drops and ointments and a seven- to 10-day course of oral antibiotics.1-8,19,20 The topical antibiotics of first choice include the fourth-generation fluoroquinolones. Excellent choices in oral antibiotics include Augmentin (amoxicillin + clavulanate, GlaxoSmithKline) 500mg BID, Keflex (cephalexin, Advancis Pharmaceutical) 500mg BID, Levaquin (levofloxacin, Johnson & Johnson) 750mg QD or 500mg BID, doxycycline 100mg BID and azithromycin 500mg PO with one dose on day one, followed by 250mg PO on days two to five.
Mild cases of dacryocystitis in children often self-resolve, while more severe forms should be managed with antibiotic therapy with consideration to dose-adjusting based upon weight. When in doubt, consultation with the pediatrician is recommended.
Surgical solutions are needed in both acute and chronic cases when conservative treatments fail.1-5,24 Dacryocystorhinostomy is the typical surgery for treating acute adult dacryocystitis.19-22 It involves resection of the bony area around the nasolacrimal canal for the purposes of gaining access to the stenotic area within the drainage system. The procedure permits the shunting of tear flow around any blockage by creating a new anastomotic passageway.1-4 The procedure is gaining popularity because it permits the surgeon to immediately drain and culture the abscess.
New treatments for dacryocystitis include endocanalicular laser and endoscopic intranasal surgical techniques.19,24 These revolutionary methods permit the cavity to be accessed without opening the entire passage.24
The patient presented here was diagnosed with acute bacterial dacryocystitis. He was prescribed topical moxifloxacin QID OD and, due to the potential of having MRSA as the potential cause, was additionally given a 10-day course of doxycycline 100mg BID, which has been shown to be an effective agent against MRSA.25 He was appointed to return in 10 days but was lost to follow-up.
Dr. Sowka is an attending optometric physician at Center for Sight in Sarasota, FL, where he focuses on glaucoma management and neuro-ophthalmic disease. He is a consultant and advisory board member for Carl Zeiss Meditec and Bausch Health.
1. Pinar-Sueiro S, Sota M, Lerchundi T, et al. Dacryocystitis: systemic approach to diagnosis and therapy. Curr Infect Dis Rep. 2012;14(2):137-46. 2/24. Pinar-Sueiro S, Fernandez-Hermida RV, Gibelalde A, et al. Study on the effectiveness of antibiotic prophylaxis in external dacryocystorhinostomy: a review of 697 cases. Ophthal Plast Reconstr Surg. 2010;26(6):467-72. 3. Paulsen FP, Thale AB, Maune S, Tillmann BN. New insight into the pathophysiology of primary acquired dacryostenosis. Opthalmology. 2001;108(12):2329-36. 4. Perry LJP, Jakobiec FA, Zakka FA, et al. Giant dacryocystomucopycele in an adult: a review of lacrimal sac enlargments with clinical and histopathologic differential diagnoses. Surv Ophthalmol. 2012;57(5):474-85. 5. Martins MC, Ricardo JR, Akaishi PM, Velasco e Cruz AA. Orbital abscess secondary to acute dacryocystitis: case report. Arq Bras Oftalmol. 2008;71(4):576-8. 6. Maheshwari R, Maheshwari S, Shah T. Acute dacryocystitis causing orbital cellulitis and abscess. Orbit. 2009;28(2-3):196-9. 7. Mauriello JA Jr, Wasserman BA. Acute dacryocystitis: an unusual cause of life-threatening orbital intraconal abscess with frozen globe. Ophthal Plast Reconstr Surg 1996;12(4):294-5. 8. Ghauri AJ, Keane PA, Scotcher SM, et al. Acute dacryocystitis associated with Epstein-Barr virus infection. Orbit. 2011;30(5):245-8. 9. Paulsen F. The human nasolacrimal ducts. Adv Anat Embryol Cell Biol. 2003;170:III-XI, 1-106. 10. Van Santvliet L, Ludwig A. Determinants of eye drop size. Surv Ophthalmol 2004;49(2):197-213. 11. Francisco FC, Carvalho AC, Francisco VF, et al. Evaluation of 1000 lacrmal ducts by dacryocystography. Br J Ophthalmol. 2007;91(1):43-6. 12. Oyster CW. The Eyelids and the lacrimal system. In: Oyster CW. The Human Eye Structure and Function. Sunderland, MA: Sinauer Associates; 1999: 291-320. 13. Cher I. Fluids of the ocular surface: concepts, functions and physics. Clin Experiment Ophthalmol. 2012;40(6):634-43. 14. Zoumalan CI, Joseph JM, Lelli GJ Jr, et al. Evaluation of the canalicular entrance into the lacrimal sac: an anatomical study. Ophthal Plast Reconstr Surg. 2011;27(4):298-303. 15. Paulsen FP, Schaudig U, Thale AB. Drainage of tears: impact on the ocular surface and lacrimal system. Ocul Surf. 2003;1(4):180-91. 16. Johnson ME, Murphy PJ. Temporal changes in the tear menisci following a blink. Exp Eye Res. 2006;83(3):517-25. 17. Sahlin S, Chen E, Kaugesaar T, et al. Effect of eyelid botulinum toxin injection on lacrimal drainage. Am J Ophthalmol. 2000;129(4):481-6. 18. Zhuang L, Sylvester CL, Simons JP. Bilateral congenital lacrimal fistulae: a case report and review of the literature. Laryngoscope. 2010;120 Suppl 4:S230. 19. Mandal R, Banerjee AR, Biswas MC, et al. Clinicobacteriological study of chronic dacryocystitis in adults. J Indian Med Assoc. 2008;106(5):296-8. 20. McKeag D, Kamal Z, McNab AA, Sheorey H. Combined coliform and anaerobic infection of the lacrimal sac. Clin Exp Ophthalmol. 2002;30(1):52-4. 21. Luo B, Li M, Xiang N, et al. The microbiologic spectrum of dacryocystitis. BMC Ophthalmol. 2021;21(1):29. 22. Chen L, Fu T, Gu H, et al. Trends in dacryocystitis in China: a STROBE-compliant article. Medicine (Baltimore). 2018;97(26):e11318. 23. Deangelis D, Hurwitz J, Mazzulli T. The role of bacteriologic infection in the etiology of nasolacrimal duct obstruction. Can J Ophthalmol. 2001;36(3):134-39. 24. Saeed BM. Endoscopic DCR without stents: clinical guidelines and procedure. Eur Arch Otorhinolaryngol. 2012;269(2):545-9. 25. Bhambri S, Kim G. Use of Oral Doxycycline for community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) infections. J Clin Aesthet Dermatol. 2009;2(4):45-50. |


