Over the past several decades, optometrists have moved from the sidelines into a more integral role when it comes to providing care for diabetes patients. Given optometrists’ position as frontline healthcare providers, our involvement in diabetes management should begin, not end, at a patient’s initial diagnosis.
Many patients with prediabetes or type 2 diabetes believe they have an irreversible hereditary condition. While this may be the case for those with type 1 diabetes, a completely different story exists for patients with type 2 diabetes.
This article discusses the optometrist’s expanding role in managing type 2 diabetes (and prediabetes) and offers different lifestyle recommendations that can help patients manage their health and overcome potential long-term diabetic ocular complications. While these interventions do not replace standard-of-care diabetes management, they can prevent the onset and slow the progression of diabetic retinopathy (DR) and serve as an adjunct to the breakthrough treatments we’re continuing to see.
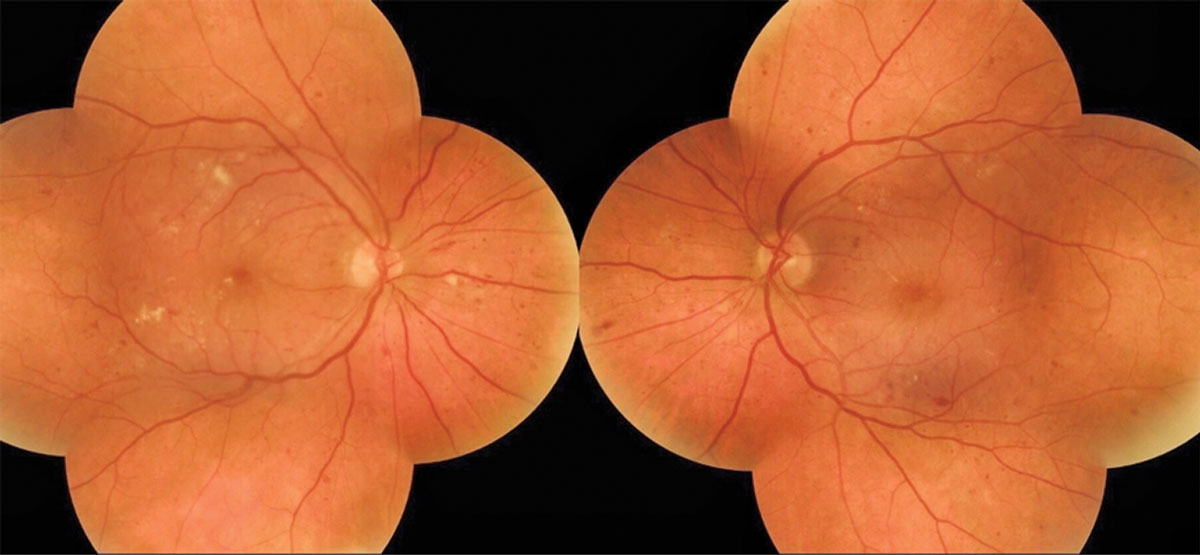 |
| A 58-year-old female presented for her first eye exam without knowing she had type 2 diabetes and was found to have diabetic retinal changes OU. Click image to enlarge. |
Prevalence and Cost
According to the CDC, more than 34 million US adults have diabetes, seven million of whom are undiagnosed.1 The number of Americans diagnosed with diabetes has almost doubled over the past 20 years, with 95% of cases being type 2 diabetes.1 Optometrists alone diagnose type 2 diabetes in more than a quarter-million patients each year based on our eye exams.2 After considering the fact that one in three adults has prediabetes, we can see that almost half of the US adult population is at risk of sight-threatening retinopathy, morbidity and mortality, as diabetes is among the leading causes primarily due to its association with cardiovascular disease.1,3
Approximately one in three diabetic patients have some form of DR, with up to 24,000 new cases occurring each year—a number that is expected to increase 40% by 2050.4-6 DR is the leading cause of vision loss among US adults.7
Each year, the US healthcare system spends $327 billion to manage diabetes, its complications and the resulting loss in productivity.8 Healthcare costs for diabetes patients are more than double those of unaffected patients.8 Regardless of changes to US healthcare policy, no amount of reform can indefinitely sustain this growing economic burden.
Given these staggering statistics, this is an all-hands-on-deck scenario for healthcare providers who interact with patients who have type 2 diabetes, prediabetes or metabolic syndrome. It is no longer solely the responsibility of the primary care provider (PCP) or dietician to discuss the importance of lifestyle intervention.
Start the Conversation
When discussing lifestyle intervention with patients, it’s crucial to meet them where they’re comfortable and tailor the conversation in a way that is manageable so they can engage in and benefit from the conversation. Asking open-ended questions can help start and continue the dialogue in a non-threatening way. For ODs with access to a patient’s lab work, mentioning their last A1c and including specific results can be another beneficial conversation starter. Have printed resources available to patients that they can reference on their own time.
Patients who are more motivated to make the necessary dietary and lifestyle changes are likely to respond more proactively and positively to discussion. Some patients may feel uncomfortable with change or frustrated with conflicting information or slow results and find it harder to commit to a healthier lifestyle. Motivate them to stick it out and put in the work now so it pays off later.
Start by identifying one area for the patient to focus on and provide tangible steps for them to work toward. Encourage habits that are feasible for them, and ask them to determine how a healthier lifestyle would benefit them personally. Making this connection could ultimately lead to a more positive outcome.
It can also be useful to have patients keep a journal of their fasting and postprandial blood sugars so they can track exactly what works for them (and what doesn’t). This can help provide clarification for those who may otherwise be overwhelmed or unsure of where to start.
These patients must understand that improving their retinopathy, blood sugar and overall health is a slower process, with diabetic retinal changes taking more than six weeks to improve in most cases, but failing to do so could have destructive effects. It can help to check in with patients periodically to recap previous conversations and provide continued encouragement, all while building a good rapport.
Patients should be advised to collaborate with their PCP prior to engaging in any dietary or lifestyle intervention, as modification to insulin dosage or other medications may be necessary.
Here are five changes to advocate for all patients, but vulnerable patients in particular:
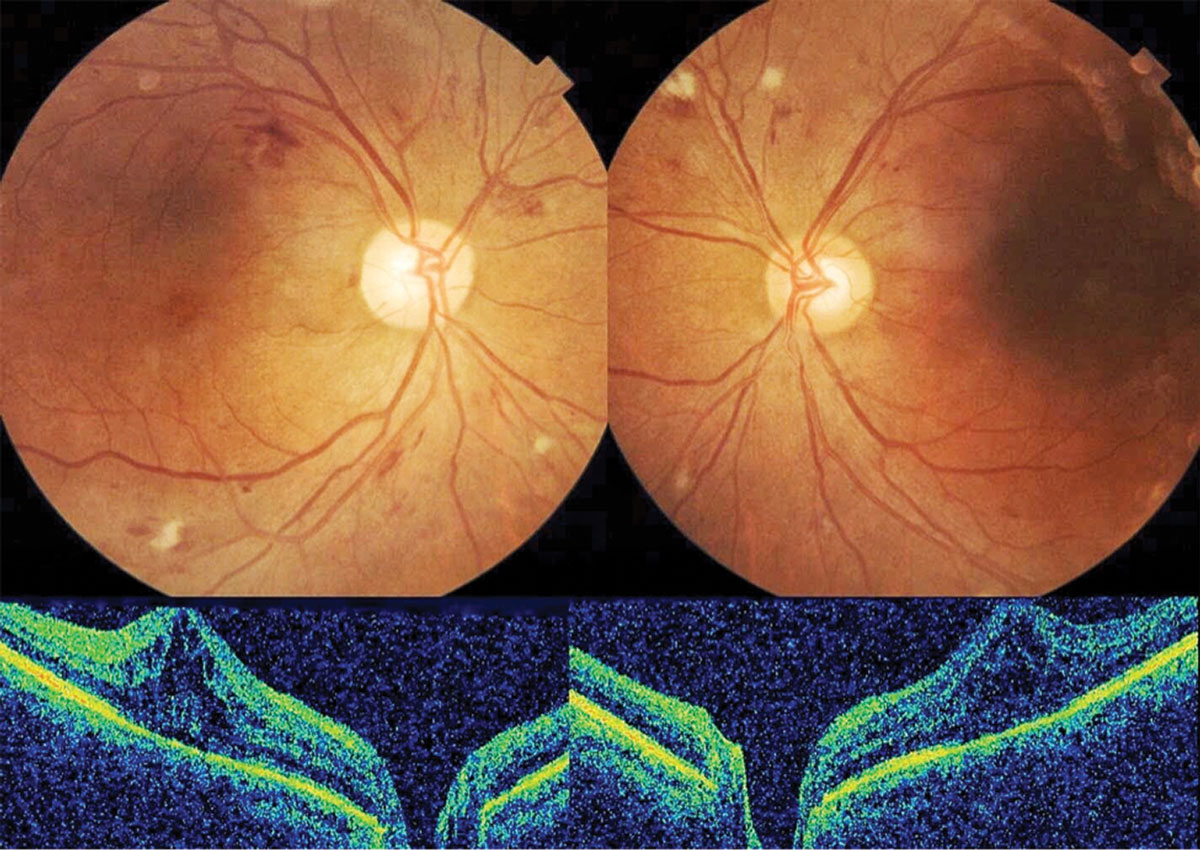 |
| A 66-year-old female had a history of uncontrolled type 2 diabetes and severe nonproliferative retinopathy with diabetic macular edema OU. Click image to enlarge. |
1. Cut Sugar Consumption
One of the first topics to address is excessive sugar consumption, including fructose and artificial sweeteners.9-14 In 1822, consumption of added sugars in the American diet was equivalent to one 12oz can of soda every five days.11 Today, the average American consumes this amount of sugar every seven hours.11 Do not assume patients have already addressed excess sugar consumption in an effort to control their diabetes and optimize their health.
Fructose consumption often flies under the radar for patients trying to make healthy dietary changes. Average fructose consumption in the United States exceeds 50g per day and is higher among adolescents.12 Excess fructose consumption is directly associated with elevated fasting blood glucose levels, hyperinsulinemia, metabolic syndrome and cardiovascular disease.13,14 Limiting daily fructose intake to less than 20g per day (the equivalent of 1.5 apples) and avoiding products containing high-fructose corn syrup are good starting points for patients with metabolic problems, including type 2 diabetes.13
2. Limit Eating Time
Intermittent fasting and time-restricted eating (TRE) were common practices over 100 years ago in the pre-insulin days that were used by doctors to optimize health and longevity for their diabetic patients. Newer research shows that TRE remains promising as an effective adjunct therapy for controlling blood glucose levels in type 2 diabetes.
TRE can optimize insulin resistance, fasting blood glucose level, body composition and circadian rhythm.15 TRE also improves cardiovascular biomarkers, such as total cholesterol, triglycerides, blood pressure and high sensitivity C-reactive protein (hs-CRP). The recent literature on meal timing is so promising that the American Heart Association advocates for it to optimize cardiometabolic health.16
The typical daily feeding window exceeds 15 hours on average.17 In TRE, meal-timing is limited to an eight- to 12-hour window. Essentially, the patient’s daily caloric intake remains the same, but breakfast is pushed later and dinner is pushed forward. Only one in 10 adults habitually maintains a 12-hour fasting window every day.15
A recent study looking at TRE in patients with metabolic syndrome found that timing meals within a 10-hour window (allowing a 14-hour nightly fast) over 12 weeks had the most favorable outcomes on many cardiometabolic markers.15 These included improved insulin resistance, body mass index, low-density lipoprotein cholesterol and blood pressure.15 Hemoglobin A1c was reduced by almost 1%, and liver enzymes, which are classic in non-alcoholic fatty liver disease, were reduced by roughly 10%.15 Diet quality and physical activity remained stable.15 No adverse events were reported.15
TRE may arguably be the easiest lifestyle intervention for patients to understand and implement, as they do not have to learn and adhere to a new diet or meal plan. This is the best option for patients who would rather change not what they eat, but when they eat.
3. Cut the Carbs
This is probably where you’ll encounter the most resistance, but it cannot be ignored. The literature on the efficacy of dietary intervention in type 2 diabetes is vast and conflicting at times. Regardless of which nutritional approach patients adopt, studies seem to consistently demonstrate a direct relationship between carbohydrate restriction (a daily glycemic carbohydrate intake less than 45% of total calories) and improvements in insulin resistance and A1c.18 Cardiovascular risk factors, including total cholesterol, triglycerides, blood pressure and hs-CRP, also significantly improve with carbohydrate restriction.19 Tracking apps, such as “MyFitnessPal,” are useful tools for helping patients understand their daily intake and identify where their calories are coming from.
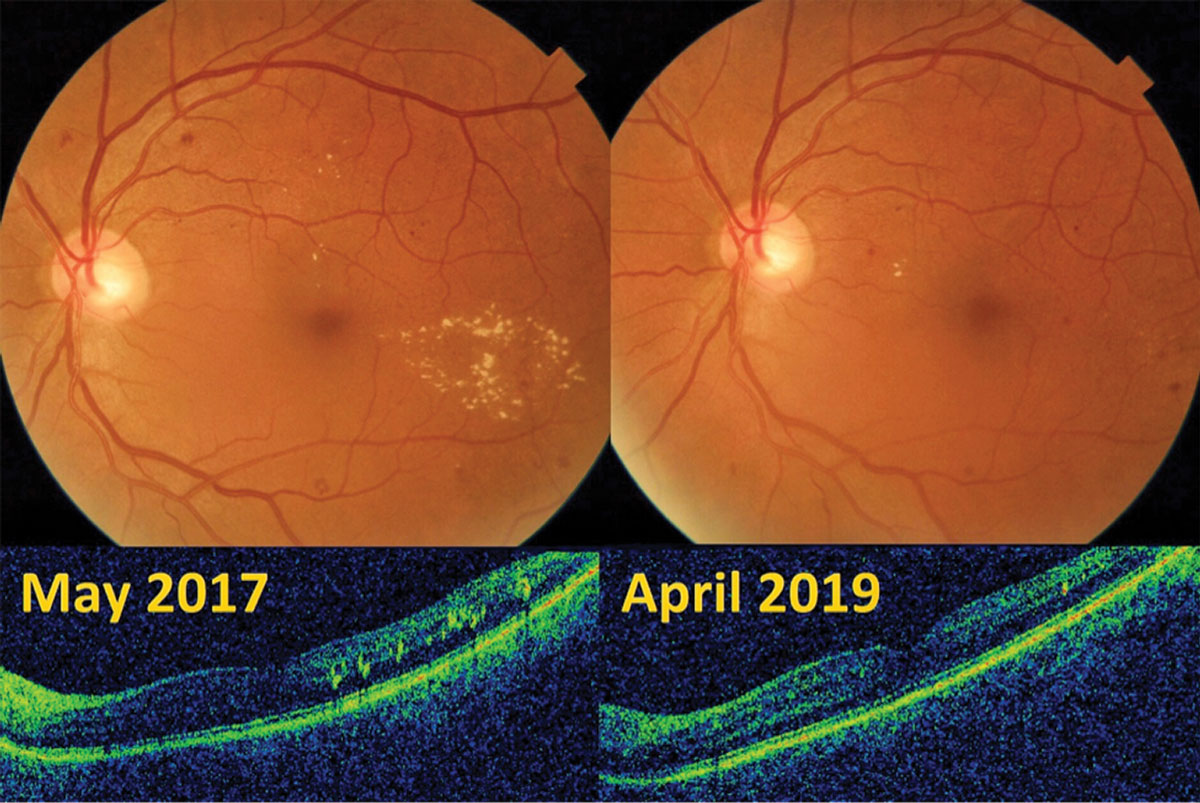 |
| Mild diabetic retinopathy OS with early parafoveal macular edema in a 62-year-old male improved with TRE and carbohydrate restriction. Click image to enlarge. |
One of the most heavily researched nutritional interventions for carbohydrate restriction is the Mediterranean-style dietary approach. By definition, the Mediterranean diet is lower in carbohydrates and higher in healthy fats.20 Studies consistently show improvements in glycemic control, weight loss, hemoglobin A1c and other cardiovascular risk factors with this diet.20 The Mediterranean diet is more efficacious than both low-fat and vegetarian-style dietary interventions for type 2 diabetes.21 Other popular low-carb approaches include the paleo, whole30 and ketogenic diets.
Using telemedicine to educate patients with type 2 diabetes on the benefits of sustainable carbohydrate restriction, one company’s recent two-year trial reported a remission rate in diabetes of approximately 7% and an average A1c reduction of 0.9%.19 This is encouraging, given that less than 2% of patients with type 2 diabetes achieve long-term remission with current standard-of-care management.22 By contrast, one in three patients with type 2 diabetes who undergo bariatric surgery achieve long-term remission.23
4. Optimize Sleep Schedule
Consistent, quality sleep is one of the most underrated factors when it comes to metabolic health. Getting seven to eight hours of sleep per night is crucial for patients looking to manage their type 2 diabetes and overall health.24 The risk of developing or worsening diabetes increases with sleep durations outside this range.24
When patients report poor quality sleep, it opens the door for optometrists to discuss potential underlying issues, such as sleep apnea and excessive exposure to blue light at night. Sleep apnea can be a common comorbidity in metabolic syndrome, diabetes and glaucoma, so referring appropriate patients for a sleep study could have a significant impact on their health. Explaining that blue light can cause circadian rhythm disruption and melatonin suppression can open the conversation up to the importance of blue-blocking lens technologies or apps for use before bedtime if screen time is unavoidable.25,26
5. Value Consistent Exercise
The discussion would be incomplete without addressing the importance of regular exercise and movement. Some patients may feel discouraged by or overwhelmed with a new fitness routine. It is important to convey that even basic exercises, such as walking for 20 minutes per day, can significantly benefit patients’ metabolic health.27 Consistency matters more than intensity of exertion.
Comorbidities (e.g., rheumatoid arthritis, obesity) may prevent patients from engaging in prolonged periods of weight-bearing movement. Non-weight-bearing exercises such as swimming, aqua aerobics and stationary cycling are viable alternatives. Remind proactive patients that whatever they enjoy doing to stay active will likely be the most sustainable routine for them moving forward.
Depending on practice modality, office location and insurance plan, some patients may even be eligible for discounted or free gym memberships. Share this with patients so they know their options.
As integral members of the healthcare team, optometrists have been encountering a growing number of patients presenting with uncontrolled (and undiagnosed) metabolic diseases, such as type 2 diabetes. No longer can we defer to a patient’s other healthcare providers to discuss the importance of evidence-based behavioral changes in managing their health. By educating patients on the risk of permanent vision loss due to their diabetes, we can help them lessen the risk of or altogether avoid long-term ocular complications.
Dr. Cornwell graduated from the New England College of Optometry in 2015 and completed a residency in ocular disease with the Indian Health Service. He works at a rural community health center in northern California where he provides eye care to populations in need and helps patients manage ocular manifestations of various systemic diseases.
1. CDC. National Diabetes Statistics Report, 2020. www.cdc.gov/diabetes/library/features/diabetes-stat-report.html. Accessed June 4, 2020. 2. AOA. 21st-century optometric care for the 21st-century pandemic. www.aoa.org/news/clinical-eye-care/21st-century-optometric-care. Accessed June 4, 2020. 3. CDC. National Diabetes Statistics Report, 2017. dev.diabetes.org/sites/default/files/2019-06/cdc-statistics-report-2017.pdf. Accessed June 4, 2020. 4. Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond). 2015;2:17. 5. CDC. Economic studies. www.cdc.gov/visionhealth/projects/economic_studies.htm. Accessed June 4, 2020. 6. NEI. Diabetic retinopathy data and statistics. www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/diabetic-retinopathy-data-and-statistics. Accessed June 4, 2020. 7. CDC. Watch out for diabetic retinopathy. www.cdc.gov/features/diabeticretinopathy/index.html. Accessed June 4, 2020. 8. ADA. Economic costs of diabetes in the U.S. in 2017. care.diabetesjournals.org/content/early/2018/03/20/dci18-0007. Accessed June 4, 2020. 9. Fagherazzi G, Vilier A, Sartorelli DS, et al. Consumption of artificially and sugar-sweetened beverages and incident type 2 diabetes in the Etude Epidemiologique Aupres Des Femmes De La Mutuelle Generale De L’Education Nationale-European Prospective Investigation into Cancer and Nutrition Cohort. Am J Clin Nutr. 2013;97(3):517-23. 10. Nettleton JA, Lutsey PL, Wang Y, et al. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-ethnic Study of Atherosclerosis (MESA). Diabetes Care. 2009;32(4):688-94. 11. Guyenet S. By 2606, the US diet will be 100 percent sugar. Whole Health Source. February 18, 2012. wholehealthsource.blogspot.com/2012/02/by-2606-us-diet-will-be-100-percent.html. Accessed June 4, 2020. 12. Vos MB, Kimmons JE, Gillespie C, et al. Dietary fructose consumption among US children and adults: the third National Health and Nutrition Examination Survey. Medscape J Med. 2008;10(7):160. 13. Kelishadi R, Mansourian M, Heidari-Beni M. Association of fructose consumption and components of metabolic syndrome in human studies: a systematic review and meta-analysis. Nutrition. 2014;30(5):503-10. 14. Brown CM, Dulloo AG, Montani JP. Sugary drinks in the pathogenesis of obesity and cardiovascular diseases. Int J Obes. 2008;32:S28-34. 15. Wilkinson MJ, Manoogian EMC, Zadourian A, et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020;31(1):92-104. 16. St-Onge MP, Ard J, Baskin ML, et al. Meal timing and frequency: implications for cardiovascular disease prevention: a scientific statement from the American Heart Association. Circulation. 2017;135(9):e96-121. 17. Gupta NJ, Kumar V, Panda S. A camera-phone based study reveals erratic eating pattern and disrupted daily eating-fasting cycle among adults in India. PloS One. 2017;12(3):e0172852. 18. Snorgaard O, Poulsen GM, Andersen HK, et al. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Res Care. 2017;5(1):e000354. 19. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. Frontiers. June 5, 2019. www.frontiersin.org/articles/10.3389/fendo.2019.00348/full#B4. Accessed June 4, 2020. 20. Huo R, Du T, Xu Y, et al. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: a meta-analysis. Eur J Clin Nutr. 2015;69(11):1200-8. 21. Schwingshackl , Chaimani A, Hoffmann G, et al. A network meta-analysis on the comparative efficacy of different dietary approaches on glycaemic control in patients with type 2 diabetes mellitus. Eur J Epidemiol. 2018;33(2):157-70. 22. Karter AJ, Nundy S, Parker MM, et al. Incidence of remission in adults with type 2 diabetes: the diabetes & aging study. Diabetes Care. 2014;37(12):3188-95. 23. Sjöström Lars, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311(22):2297-304. 24. Shan , Ma H, Xie M, et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2015;38(3):529-37. 25. Chang AM, Aeschbach D, Duffy JF, et al. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci USA. 2015;112(4):1232-7. 26. Green A, Cohen-Zion M, Haim A, et al. Evening light exposure to computer screens disrupts human sleep, biological rhythms, and attention abilities. Chronobiol Int. 2017;34(7):855-65. 27. Qiu S, Cai X, Schumann U, et al. Impact of walking on glycemic control and other cardiovascular risk factors in type 2 diabetes: a meta-analysis. PloS One. 2014;9(10):e109767. |

