 |
|
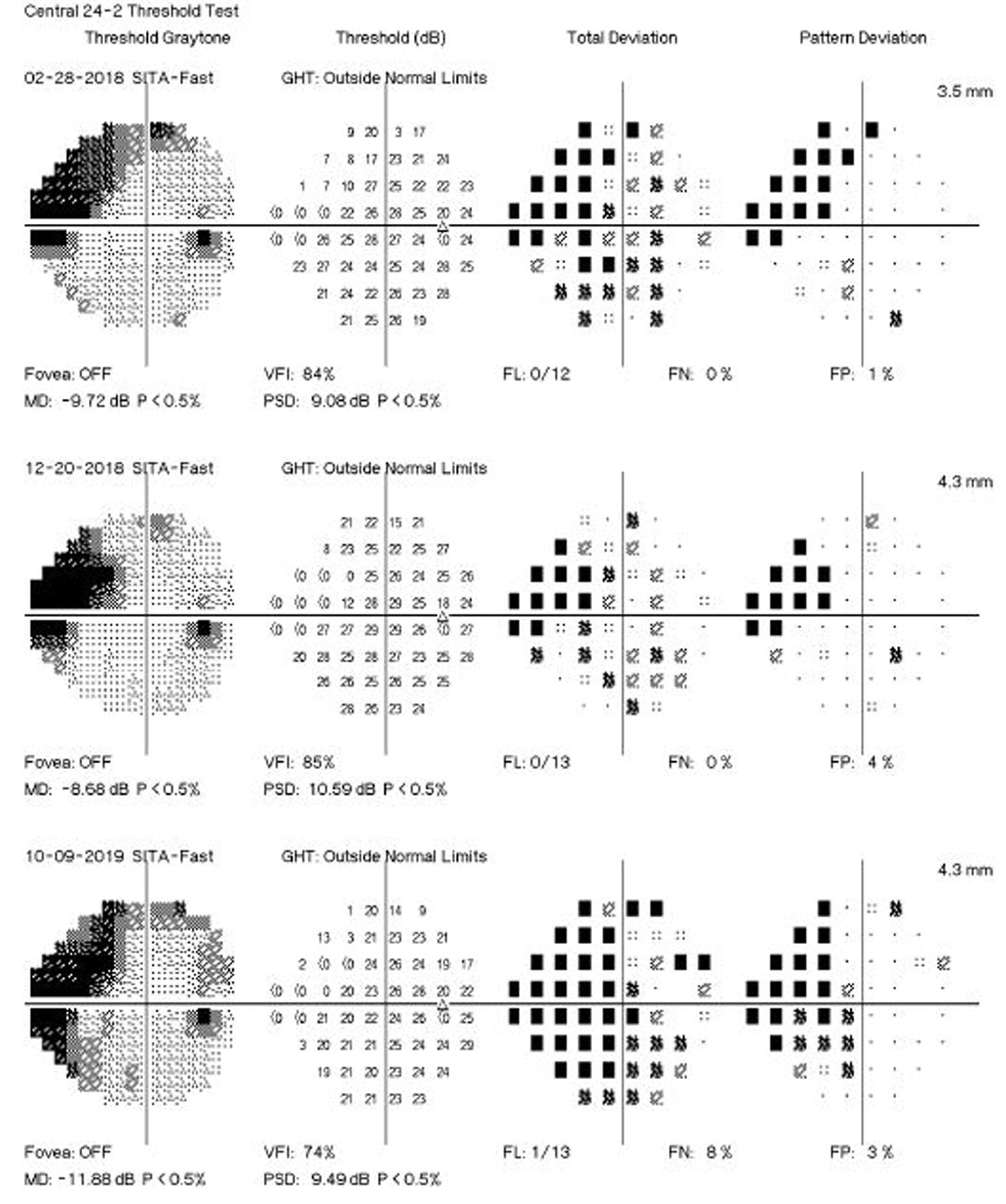
A new study’s findings give support for the use of MD change in SAP as a suitable endpoint in clinical trials investigating potential new therapies in glaucoma. Photo: Brian D. Fisher, OD. Click image to enlarge. |
Several algorithms have been proposed to identify visual field (VF) progression over time using different approaches to separate true change from test-retest variability. These approaches can be broadly divided into two categories: event- vs. trend-based methods. In event-based methods, the differences in VF sensitivities of follow-up tests are compared with those of previously established baseline exams. The commercially available Guided Progression Analysis (GPA) software from the Humphrey Field Analyzer (Carl-Zeiss Meditec) performs an analysis of change of each follow-up test compared with the average of two baseline tests, based on the pattern deviation points. A recent investigation in Ophthalmology, however, has proposed that trend-based analyses of VF data can provide estimates of rates of change over time. These estimates could potentially identify those patients with fast disease progression who could be at higher risk for developing disability from the disease.
The study included 246 eyes of 168 patients with glaucoma followed every six months for up to five years. Participants were required to have a minimum of five reliable standard automated perimetry (SAP) tests during the first two years of follow-up. Events of progression were evaluated using two methods: the GPA and a US Food and Drug Administration (FDA)-suggested endpoint. The latter is an alternative method for assessing events requiring at least five VF points showing a significant depression of at least 7dB.
The researchers showed that rates of SAP mean deviation (MD) change of glaucomatous eyes calculated over the initial two years of follow-up were strongly predictive of events of progression over subsequent years. The results were similar whether progression events were defined based on the conventional GPA algorithm or the FDA-suggested endpoint. Eyes that developed a progression endpoint had, on average, faster slopes of MD change during the initial two years of follow-up (-0.82dB/year vs. 0.08dB/year). Each 0.1dB/year faster two-year rate of MD loss was associated with a 26% increased risk of developing a GPA progression endpoint and a 32% increased risk of developing an FDA-suggested endpoint.
However, despite such benefits, the authors noted that the acceptance of rates of MD change as valid endpoints in clinical trials requires clear demonstration that such rates are predictive of clinically relevant endpoints.
“When evaluating slopes of change in individual eyes, clinicians need to be aware of the potential imprecision of such slopes and the need to obtain an adequate number of tests that will ensure enough confidence around the slope estimate,” they wrote in their paper.
Medeiros FA, Jammal AA. Validation of rates of mean deviation change as clinically relevant endpoints for glaucoma progression. Ophthalmology. December 24, 2022. [Epub ahead of print]. |

