23rd Annual Glaucoma ReportFollow the links below to read the other articles from our 23rd annual Glaucoma Report:Glaucoma Surgery: Are You Ready to Refer? Looking to the Future of Glaucoma Treatment 10-2 Visual Field Testing: A Tool for All Glaucoma Stages |
In recent years, minimally invasive glaucoma surgeries (MIGS) have exploded onto the scene to help fill a gap in the glaucoma treatment algorithm. Traditionally, first-line therapy for primary open-angle glaucoma (POAG) relied on topical glaucoma medications or selective laser trabeculoplasty to lower intraocular pressure (IOP). If those treatment options didn’t work, the patient would be referred to a glaucoma surgeon to consider more aggressive filtration surgical procedures such as trabeculectomy or tube shunts, which come with risks such as bleb-related complications, diplopia and hypotony.1,2
However, the majority of glaucoma patients in the average practice have neither completely mild nor advanced disease; rather, they lie somewhere in the middle. These patients are often taking multiple medications, are not ideally controlled and could benefit from a treatment modality more aggressive than topical medication, but less so than filtration surgery. This is where MIGS fit in.
Although MIGS offer mild to moderate glaucoma patients a low risk profile similar to cataract surgery, the question remains as to whether the procedure provides a corresponding low reward. So far, the literature continues to show that MIGS provide low risk for glaucoma patients while also lowering IOP and, arguably just as important, decreasing the medication burden on our glaucoma patients. This article discusses recent data on MIGS and the role optometry plays in the comanagement of these surgeries.
 |
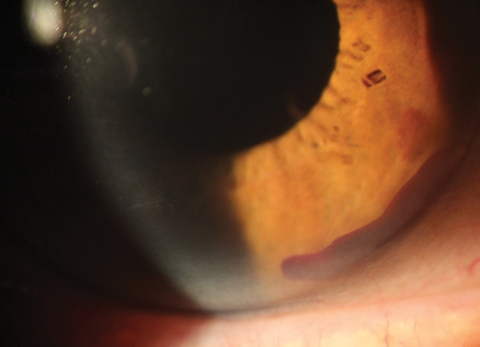
| With Cypass, a cyclodialysis cleft can be seen with gonioscopy around the edges of the device. Click image to enlarge. |
What are MIGS?
MIGS share five distinct characteristics:3
(1) ab-interno approach
(2) minimally traumatic, with little disruption of normal anatomy and physiology
(3) at least modest efficacy
(4) high safety
(5) rapid recovery
MIGS can be classified by the anatomical structure they target in an attempt to allow or decrease resistance to aqueous outflow.
Schlemm’s canal. This is accessed by stenting through the trabecular meshwork and dilating Schlemm’s canal or ablating or excising the trabecular meshwork.
Supraciliary space. This anatomical structure has an enormous surface area and is very absorptive; therefore, it carries the potential to substantially lower IOP. It is accessed by stenting, and access to this space comes with the added benefit of bypassing outflow obstructions encountered with the trabecular meshwork, specifically episcleral venous resistance.
Subconjunctival space. Like the supraciliary space, some MIGS take advantage of the subconjunctival space’s bypass of outflow obstructions involving the trabecular meshwork. Procedures that target this anatomical area are bleb-forming. Therefore, though they are more effective at lowering IOP, they carry a slightly increased risk, and some glaucoma surgeons categorize them as “MIGS plus.” The Holy Grail MIGS would produce an IOP-lowering effect similar to traditional trabeculectomy, but with the safety profile of cataract surgery.
 |
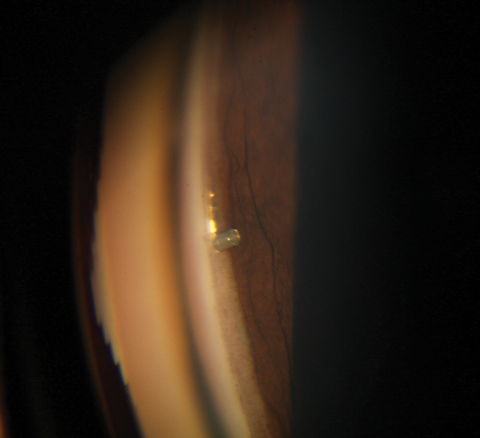
| Hyphema is one of a few postoperative complications to consider with MIGS. Click image to enlarge. |
MIGS in the Literature
Here is a look at the current research on several of the more well-known MIGS, based on their anatomical target:
Trabecular meshwork and Schlemm’s canal devices.
• Trabectome (Neomedix). Although it predates the coining of the term “minimally invasive glaucoma surgery,” this device started the MIGS revolution with FDA approval in 2004 for use independently or in conjunction with cataract surgery.4 The procedure involves ablating 120 to 180 degrees of the trabecular meshwork and the inner wall of Schlemm’s canal to lower resistance to aqueous outflow.5
A large study of 5,435 patients receiving Trabectome surgery with up to 90 months of follow-up provides some positive results for this procedure. Researchers found that, after 90 months, IOP dropped from 23.0mm Hg (+/-7.9) to 16.5mm Hg (+/-3.8), while decreasing the number of glaucoma medications needed from 2.6 (+/-1.3) to 1.6 (+/-1.3).6 They, along with other researchers, found the most common postoperative complication with Trabectome is transient hyphema.6-8
• iStent (Glaukos). Approved in 2012 for use in conjunction with cataract surgery, this device is designed to serve as a bypass through the trabecular meshwork to facilitate outflow of aqueous.7 Studies show the iStent reduces IOP and the need for glaucoma medications more than cataract surgery alone.9-11 One study revealed key points in the FDA pivotal clinical trial in regards to cataract surgery plus iStent insertion vs. cataract surgery alone.9 In the study, a 20% reduction in IOP without medication was achieved in 66% of eyes treated with cataract surgery plus an iStent vs. 48% of eyes treated with cataract surgery alone.9 In addition, twice as many patients in the cataract surgery only group were back on medications at one year compared with patients in the cataract surgery plus iStent group.9 Research also indicates the iStent is quite safe, showing minimal to essentially no added risk to the cataract procedure.9,11
 |
| The iStent acts as a bypass through the trabecular meshwork to facilitate aqueous outflow. |
Because of the relatively modest reduction in IOP found during the initial FDA study, questions still remain regarding the extent to which cataract surgery is contributing to the lowered IOP when an iStent is implanted. Recently, a retrospective study reported two-year results of 42 pseudophakic eyes implanted, off-label, with one iStent. Two years post-procedure, mean IOP dropped from 20.26mm Hg (+/- 6.00) to 13.62mm Hg (+/-4.55), with the need for fewer medications.12 Three patients experienced an IOP increase of 15mm Hg above baseline IOP, and all of them responded to topical therapy.12
Two other devices, the iStent Inject and iStent Supra are in FDA clinical trials. The iStent Inject resembles a punctal plug, with the end inserted into Schlemm’s canal and the head in the anterior chamber. This allows aqueous to flow through the lumen of the device and into Schlemm’s canal.
The iStent Supra is a supraciliary device designed to release aqueous through the uveoscleral outflow pathway. The 4mm tube is made of polyethersulfone and titanium and targets the large absorptive capacity of the suprachoroidal space.
• Ab interno canaloplasty (ABiC) (Ellex). This procedure uses an illuminated microcatheter through a corneal incision of 1.8mm to address all aspects of outflow resistance, including the trabecular meshwork, Schlemm’s canal and the collector channels. In contrast to traditional canaloplasty, ABiC does not require a tensioning suture to lower IOP.13
Recent data on 57 patients with mild to moderate POAG undergoing ABiC alone experienced a 24.4% reduction in mean IOP and a 64.5% reduction in medication use at one year postoperatively.14
• Kahook Dual Blade (New World Medical). This is a device that excises or removes the trabecular meshwork and the inner wall of Schlemm’s canal to lower resistance to aqueous outflow and enable better IOP control.15 The dual blade has demonstrated a more complete removal of trabecular meshwork compared with a microvitreoretinal blade or Trabectome, leaving behind no residual trabecular meshwork leaflets, which are prone to close.15 This allows for sustained IOP reduction and control.15
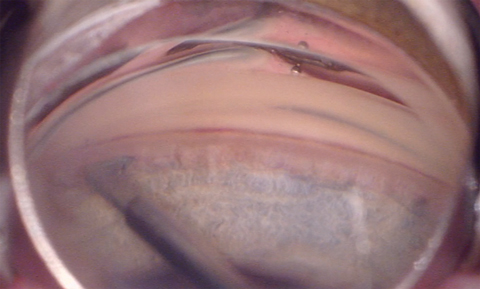 |
| The Kahook Dual Blade removes the trabecular meshwork and the inner wall of Schlemm’s canal to enable better IOP control. Click image to enlarge. |
A recent study included 120 eyes with mild, moderate and severe glaucoma treated as a standalone procedure, combined with cataract surgery or combined with other procedures.16 The results show mean IOP fell from 18.7mm Hg (+/-6.7) to 12.9mm Hg (+/-4.2) at nine months post-op.13 Glaucoma medication use dropped from 1.8 (+/-1.3) to 0.7 (+/-0.8).13 The most common postoperative complication was transient hyphema.16
• Hydrus Microstent (Ivantis). The Hydrus is an 8mm stent with the 1mm inlet segment resting in the anterior chamber, and the 7mm scaffold segment residing in Schlemm’s canal.17 The Hydrus II study shows 80% of patients that had Hydrus implantation plus cataract surgery had a 20% reduction in IOP vs. 46% in cataract surgery alone at 24 months. Additionally, 73% of patients in the combined group were medication-free compared to 38% of patients in the cataract alone group. Recently, enrollment was completed in the Hydrus IV pivotal trial, and is expected to close in a few years.
Supraciliary devices.
• Cypass Micro-Stent (Alcon). This device, approved in 2016 for use in conjunction with cataract surgery, is designed to access the supraciliary space, bypass the conventional outflow pathway and target the uveoscleral outflow path.18 The increase of aqueous through the uveoscleral outflow path has the potential to lower IOP more than devices that target compromised outflow through the trabecular meshwork, according to research.18 Once the device is implanted, a small cyclodialysis cleft is typically visible with gonioscopy around the edges of the device.
The Compass clinical trial included 505 eyes, two years of follow-up and patient randomization in a 1:3 ratio to either cataract surgery alone or cataract surgery in conjunction with Cypass implantation.18 At two years post-op, 60% of eyes that received cataract surgery alone and 77% of those who received the stent achieved a reduction in non-medicated IOP of at least 20%.18 IOP decreased by 7.4mm Hg in the stent group vs. 5.4mm Hg in the standalone cataract surgery group.18 Of the stent group, 61% had a non-medicated IOP between 6mm Hg and 18mm Hg compared with 44% in the group who received cataract surgery alone.18
Postoperative complications included transient hypotony in 2.9% of stent cases, which resolved within the first two weeks, and hyphema, which was observed in 2.7% of eyes that received the stent.18
Subconjunctival devices.
• Xen gel stent (Allergan). Approved in 2016 as a standalone procedure or in conjunction with cataract surgery, this device lowers IOP by creating a drainage pathway that takes an intrascleral course from the anterior chamber to the subconjunctival space.19 It is designed to expand and conform to the shape of the surrounding tissue after implantation.19 The device typically will be visualized in the anterior chamber with gonioscopy, with 1mm visible in the anterior chamber and 2mm visible in the subconjunctival space. A bleb will be noted on the first day postoperatively.
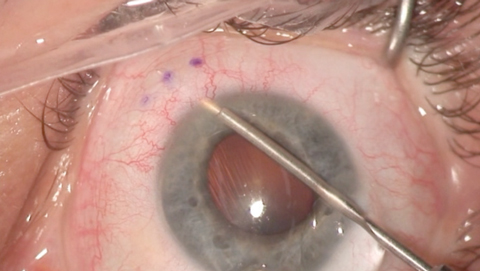 |
| The Xen gel stent creates a drainage pathway from the anterior chamber to the subconjunctival space to lower IOP. |
One study included 65 eyes of refractory glaucoma patients who experienced a failure of previous filtering or other procedures or whose IOP was unresponsive to maximally tolerated medical therapy.20 Twelve months after the Xen gel stent implantation, the mean IOP was 15.9mm Hg (+/-5.2), compared with 25.1mm Hg (+/-3.7) preoperatively. Medication use fell from 3.5 (+/-1.0) to 1.7 (+/-1.5).16 The main postoperative complications included hypotony in 16 subjects (24.6%) and 21 subjects (32.3%) requiring bleb needling.20
Postoperative Considerations
Collaborative care is common between optometry and ophthalmology in the postoperative management of cataract surgery—and the same will be true with pre- and postoperative management of MIGS. With the exception of a few additional aspects, post-op care following MIGS is essentially the same as following cataract surgery.
Gonioscopy. It is imperative to have a thorough understanding of the angle anatomy and be proficient with gonioscopy, not only to visualize the angle preoperatively to check for abnormalities and assess candidacy, but also to evaluate proper placement of MIGS devices postoperatively and identify potential problems.
Managing complications. IOP spikes, similar to those after cataract surgery, are a particular concern because glaucoma patients already suffer from a condition that has compromised the health of the optic nerve head. The severity of both the disease and the IOP spike will dictate the level of treatment.
Most of the time, these patients can be managed by adding one or two topical glaucoma medications. Aggressive tapering of postoperative steroid should be considered as well. In some cases, if the IOP spike is aggressive and early in the postoperative period, such as day one, clinicians can consider anterior decompression.
Anterior decompression is an effective way to decrease IOP rapidly, but does come with risks, namely endophthalmitis and decompression retinopathy.21,22 It should be reserved for patients presenting with emergent IOP spikes and at risk for optic nerve head damage. The patient should be placed on a topical glaucoma medication after anterior decompression and their IOP monitored for two to seven days.
With any patient experiencing IOP spikes, clinicians should establish a follow-up plan dictated by the severity of the disease and the spike.
The angle of the eye has an abundant vasculature, and it is not uncommon for a patient to experience a mild, transient hyphema in the early postoperative period. Patients with hyphema will typically present within the first few weeks with a complaint of cloudy vision. On examination of the anterior segment, the practitioner will see what appears to be an aggressive anterior chamber reaction, which in actuality is the presence of red blood cells in the anterior chamber. A small amount of blood also may be noted in the angle anatomy.
The key factor in managing a hyphema after MIGS is patient education. No intervention is needed in most cases, and practitioners should educate the patient that the cloudy vision will decrease and resolve over the next week. In the case of a large hyphema, the patient may need to be referred back to the surgeon to have an anterior chamber washout.
Placing stents in the angle of the eye puts the stent in close proximity to the iris. Although uncommon, a tuft of iris can occasionally obstruct the lumen of a stent. If the stent is obstructed on gonioscopy and IOP is elevated, clinicians should refer the patient back to the surgeon to remove the obstruction.
The rate of hypotony is low with most MIGS, as many of them do not bypass episcleral venous pressure, which means IOP won’t decrease below 10mm Hg. The exceptions are devices acting via the supraciliary and subconjunctival spaces.
In two FDA clinical trials involving supracilary and subconjunctival devices, hypotony was defined as an IOP less than 6mm Hg at any time during the study. In one study involving a supraciliary device, the rate of hypotony was minimal at 2.9%.18 Research involving a subconjunctival device found 16 out of 57 eyes (24.6%) had hypotony.20 Out of the 16 eyes that suffered hypotony, only two needed intervention; the other eyes resolved on their own.20
Optometrists managing these devices need to observe the anterior chamber to make sure it is deep and there is no iridocorneal touch. A fundus exam will rule out choroidal effusion. If the anterior chamber is formed and no choroidal folds exist, the optometrist can monitor the patient without a referral back to the surgeon. If the anterior chamber is flat or shallow, or if iridocorneal touch or choroidal folds exist, they must refer the patient to the surgeon for an anterior chamber reformation. Finally, in the setting of hypotony, it is important for patients to discontinue use of their topical glaucoma medications.
New baselines. Clinicians must establish a new baseline IOP once IOPs have stabilized following MIGS. This postsurgical IOP will become the new baseline used to monitor the patient and make decisions about the need for future treatment. Clinicians should also obtain new visual fields and retinal nerve fiber layer analysis after MIGS to better monitor the patient for
progression.
The number of patients diagnosed with glaucoma worldwide is expected to exceed 70 million by 2020, meaning optometrists will be managing more and more of these patients.23 MIGS are an important addition to the glaucoma treatment armamentarium, filling the gap between topical treatment and more invasive, traditional filtration surgeries. They can be helpful for some patients, and optometrists must be comfortable with their postoperative and long-term management. MIGS procedures open up new possibilities for the patient to be managed prior to (and possibly supplant) tubes and trabs, and the treatment landscape will continue to evolve as other devices become available. It is an exciting opportunity for optometry to be involved in the management of glaucoma.
Dr. Schweitzer practices at Vance Thompson Vision in Sioux Falls, SD, and is an adjunct clinical professor at the Illinois College of Optometry.
Financial Disclosures: Dr. Schweitzer receives consultation or lecture fees from the following companies: Allergan, Bausch + Lomb, Glaukos, Biotissue, Alcon, TearScience and Reichert.
| 1. DeBry PW, Perkins TW, Heatley G, et al. Incidence of late onset bleb-related complications following trabeculectomy with mitomycin. Arch Ophthalmol. 2002;120:297-300. 2. Edmunds B, Thompson JR, Salmon JF, Wormald RP. The National Survey of Trabeculectomy III. Early and late complications. Eye (Lond). 2002;16:297-303. 3. Saheb H, Ahmed, IIK. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23(2):96-104. 4. Administration FDA. FDA 510 (k) Database Entry Neomedix NMX-1000 (Trabectome). 2012. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=K040584. AccessedJune 28, 2017. 5. Minckler DS, Baerveldt G, Alfaro MR, Francis BA. Clinical results with the Trabectome for treatment of open-angle glaucoma. Ophthalmology. 2005;112:962-7. 6. Mosaed S. The first decade of global Trabectome outcomes. Eur Ophthalmic Rev. 2014;8(2):113-19. 7. Kaplowitz K, Bussel II, Honkanen R, et al. Review and meta-analysis of ab-interno trabeculectomy outcomes. Br J Ophthalmol. 2016;100(5):594-600. 8. Maeda M, Watanabe M, Ichikawa K. Evaluation of Trabectome in open-angle glaucoma. J Glaucoma. 2013;22(3):205-8. 9. Samuelson TW, Katz L, Wells J, et al. Randomized evaluation of the trabecular micro-bypass stent with phaco-emulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118(3):459-67. 10. Fea AM. Phacoemulsification versus phacoemulsification with micro-bypass stent implantation in primary open-angle glaucoma: randomized double-masked clinical trial. J Cataract Refract Surg. 2010;36(3):407-12. 11. Craven ER, Katz LJ, Wells JM, Giamporcaro JE. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012;38:1339-45. 12. Ferguson TJ, Berdahl JP, Schweitzer JA, Sudhagoni R. Evaluation of a trabecular micro-bypass stent in pseudophakic patients with open-angle glaucoma. J Glaucoma. 2016;25(11):896-900. 13. Lewis RA, von Wolff K, Tetz M, et al. Canaloplasty: three-year results of circumferential viscodilation and tensioning of Schlemm’s canal using a microcatheter to treat open-angle glaucoma. J Cataract Refract Surg. 2011;37:682-690. 14. Khaimi M. Twelve-month follow-up of ab interno canaloplasty as a standalone treatment and in adjunct to cataract surgery for the treatment of primary open-angle glaucoma. Poster presented at the 27th Annual AGS Meeting, March 9, 2017; Coronado, CA. 15. Seibold LK, Soohoo JR, Ammar DA, Kahook MY. Preclinical investigation of ab interno trabeculectomy using a novel dual-blade device. Am J Ophthalmol. 2013;155(3):524-9. 16. Greenwood MD, Abdullah S, Radcliffe NM, et al. A novel dual blade device for goniotomy: 9 month follow up. Poster present at ASCRS/ASOA Congress & Symposium, May 5-9, 2017; Los Angeles. 17. Pfeiffer N, Garcia-Feijoo J, Martinez-de-la-Casa JM, et al. A randomized trial of a Schlemm’s canal microstent with phacoemulsification for reducing intraocular pressure in open-angle glaucoma. Ophthalmology. 2015;122(7):1283-93. 18. Vold S, Ahmed, II, Craven ER, et al. Two-year COMPASS trial results: supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataracts. Ophthalmology. 2016;123:2103-12. 19. Lewis RA. Ab interno approach to the subconjunctival space using a collagen glaucoma stent. J Cataract Refract Surg. 2014;40(8):1301-6. 20. Allergan. Xen Gel Stent: Established Effectiveness. www.xengelstent.com/clinical-efficacy. Accessed June 28, 2017. 21. Lee S, Lee J, Kim S. Multiple retinal hemorrhage following anterior chamber paracentesis in uveitic glaucoma. Korean J Ophthalmol. 2006;20:128-30. 22. Rao S, Greenberg P, Macintyre R, Ducharme J. Ocular decompression retinopathy after anterior chamber paracentesis for uveitic glaucoma. Retina. 2009;29:280-1. 23. Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081-90. |

