23rd Annual Glaucoma ReportFollow the links below to read the other articles from our 23rd annual Glaucoma Report:10-2 Visual Field Testing: A Tool for All Glaucoma Stages Looking to the Future of Glaucoma Treatment Mastering MIGS: Today and Tomorrow |
While optometrists can serve the needs of most patients with glaucoma, many will eventually require a referral to a glaucoma specialist. The most frequent reasons for a consultation are for surgical care, diagnostic dilemmas and complex or unusual cases.
The timing of surgical consults, however, can be tricky, as the need may arise early or late in the course of open-angle glaucoma (OAG), depending on the case. In addition, optometrists should be prepared for patients susceptible to angle closure.
Here, we discuss the available surgical options for glaucoma, when optometrists should consider a referral and post-op care.
Mild to Moderate OAG
Laser trabeculoplasty, which includes argon laser trabeculoplasty and selective laser trabeculoplasty (SLT), is a common treatment option for patients with mild to moderate OAG. SLT involves the application of a low energy, Q-switched, frequency-doubled Nd:YAG laser (532nm) to the trabecular meshwork (TM).1 Researchers speculate the laser energy “selectively” targets pigmented TM cells with minimal collateral thermal damage.2,3 Studies also indicate the mechanism of action of SLT is low-level inflammation that recruits macrophages to clear debris from and increase aqueous outflow through the TM.2,3
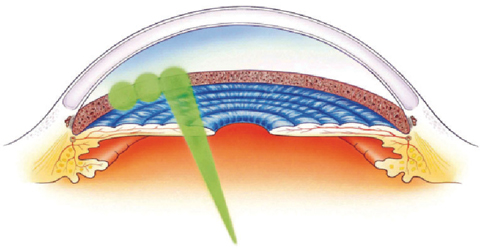 |
| For SLT, large spots of a low-energy laser are applied to the trabecular meshwork. Image: Ellex, Inc. |
Success, defined as no less than a 20% decrease in intraocular pressure (IOP) following primary SLT, is achieved in approximately 70% of eyes at six months and 60% to 95% at 12 months.4-10 The benefits of SLT are known to wane with time such that about half of eyes will lose the IOP-lowering effect by two years.4-10 SLT may also blunt diurnal IOP fluctuation, especially at night.2,11 Because SLT does not physically alter the TM, it theoretically can be repeated as necessary.
When to refer. SLT is indicated for the treatment of mild to moderate OAG. Its excellent safety profile, ability to lower IOP by 20% to 30% in most patients and repeatability make it a good choice as first-line therapy for many patients.4,6,7,12-15 SLT may be employed as an adjunct therapy in eyes with uncontrolled or progressive glaucoma already being treated with topical medical therapy or as replacement therapy to reduce the number of medications or improve compliance.
Nearly any form of OAG with an intact and gonioscopically visible TM is amenable to SLT. This includes primary OAG (POAG), ocular hypertension, secondary OAGs such as pigment dispersion and pseudoexfoliation, normal tension glaucoma and steroid-induced glaucoma.1,2,4 Contraindications include angle-recession, congenital/developmental and neovascular glaucomas. Inflammatory glaucomas are also contraindicated due to risk of post-op inflammation.1,2,4
Pretreatment IOP is generally considered the best predictor of SLT success, with greater baseline IOPs resulting in greater post-SLT IOP reductions.4,16 Studies have also correlated the density of angle pigment with SLT efficacy, although patients with even modest TM pigment often achieve significant reduction.4,17 Therefore, ideal SLT candidates have IOPs in the upper twenties and at least moderate TM pigment.
Postoperative care. For most eyes, a substantial IOP response appears at least six weeks following SLT.18 Thus, patients should continue their glaucoma medications postoperatively until a response is seen.
Most clinicians do not prescribe topical anti-inflammatory medications following SLT because allowing postoperative inflammation to run its course naturally may enhance the effectiveness of the procedure.
SLT is generally safe with a low risk profile, but complications can occur. Perhaps the greatest risk is simply that the procedure will fail. An IOP spike, occurring in approximately 4% to 5% of eyes, is often transient and rarely requires surgical intervention.19 Mild iritis is common within the first few days following SLT and may affect up to 83% of eyes, but is often self-limiting and can be observed.19 In symptomatic patients, clinicians can consider topical NSAIDs or steroids to alleviate any discomfort. Other, less frequent complications include hyphema, macular edema, corneal haze, refractive error shift, peripheral anterior synechiae and choroidal effusion.19
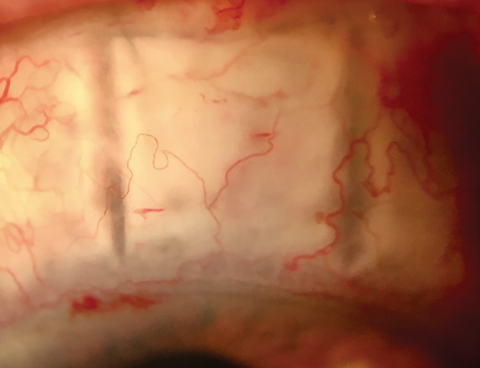 |
| Variations of trabeculectomy, such as non-penetrating deep sclerectomy, seen here early post-op, further improve surgical outcomes for patients with advanced disease.38 Click image to enlarge. |
MIGS
A variety of minimally invasive glaucoma surgeries (MIGS) are now available for patients with mild to moderate glaucoma. These procedures share many of the benefits of SLT, but also have one important disadvantage—the surgeon must enter the globe, creating additional risks such as infection and hemorrhage. To minimize this additional risk, most MIGS are performed at the time of cataract extraction.
When to refer. While cataract surgery alone often improves IOP control, adding a MIGS procedure can further lower postoperative IOP and decrease dependence on topical medications. The specific clinical indications and patient selection criteria vary among procedures, but most patients with mild to moderate POAG in need of cataract surgery are candidates for MIGS.
For instance, the iStent (Glaukos) device is placed into Schlemm’s canal during cataract surgery to enable aqueous to bypass the high resistance of the TM and flow directly into the canal.20 A more recent MIGS innovation is the ab interno canaloplasty (ABiC), which restores the natural outflow pathways without the formation of a bleb.20 Preliminary reports indicate it can lower IOP by approximately 30%.21 ABiC is one of the few MIGS procedures approved for use outside cataract surgery in the United States.
Endocyclophotocoagulation (ECP) is a cyclodestructive procedure that is delivered internally using a diode laser and an endoscope that allows direct visualization of the ciliary processes—creating minimal collateral damage.22 Ablation of the ciliary processes results in decreased aqueous production and reduced IOP. Investigators found that, when performed on patients with mild to moderate OAG, ECP in conjunction with phacoemulsification decreased IOP by at least 20% in about 60% of eyes.23 Phacoemulsification alone in patients with POAG lowers IOP by only 13%.24
Postoperative care. Because MIGS is typically performed in conjunction with cataract extraction, the postoperative care process is essentially the same as that following conventional cataract surgery.
The addition of MIGS may increase the risk of certain complications such as hyphema. However, the less invasive nature of MIGS tends to produce fewer postoperative complications compared with traditional glaucoma surgery.
A key element of the care process for MIGS is re-evaluating the patient’s glaucoma medications. Most patients require fewer medications to control their IOP following MIGS. Some clinicians choose to stop all glaucoma medications one month prior to surgery and then restart them as needed afterward. This strategy aims to minimize the risk of postoperative hypotony. Others will defer adjusting medications until after surgery, based upon the observed IOP lowering effect of the procedure.
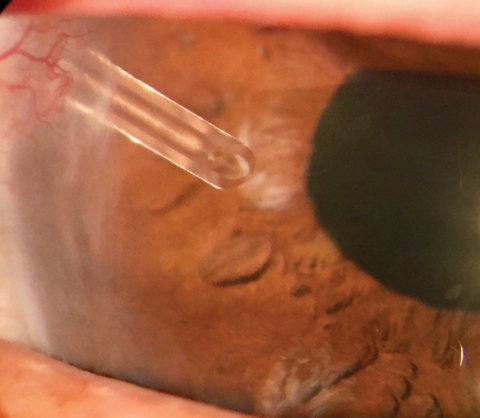 |
| When a glaucoma drainage device is positioned in the anterior chamber, aqueous drains through the tube to a reservoir on the ocular surface. |
Advanced Glaucoma
Trabeculectomy is the most frequently performed surgical procedure for late-stage glaucoma. It creates an alternative outflow pathway for aqueous and results in the formation of a filtering bleb under the conjunctiva where the aqueous accumulates and is gradually absorbed by the tissue.25 A successful trabeculectomy often results in IOPs in the high single digits to low mid-teens without use of medication.25
When to refer. Clinicians must weigh many variables before referring a patient for trabeculectomy, including severity of disease, target IOP, rate of progression and life expectancy. In general, incisional surgery is indicated when medical and laser therapy has failed to adequately control IOP.25
Careful assessment of the rate of progression is key to identifying patients who may benefit from referral for trabeculectomy.
Although central fixation is often spared until late in the course of the disease, patients whose central visual field (VF) becomes involved early, possibly influencing the risk of decreased visual acuity and blindness, may benefit from early referral for surgery.26,27
With improvements in surgical outcomes, clinicians can consider a patient a candidate for trabeculectomy after failing to adequately control IOP with two to three drugs.28
Some particularly aggressive forms of glaucoma are difficult, or even impossible, to manage successfully without surgery, increasing the importance of prompt referral. Examples include patients with neovascularization or synechial closure of the chamber angle, iridocorneal endothelial syndrome and most lens-associated glaucomas.
Postoperative care. The long-term success of filtering surgery depends on appropriate postoperative care. In the immediate postoperative period, steroids are tapered over eight to 12 weeks, or longer as needed, to control inflammation. Cycloplegics are prescribed for two to three weeks after surgery to maintain anterior chamber depth and prevent synechia. Broad-spectrum antibiotics are used for the first two weeks after surgery to prevent infection.
Following filtration surgery, IOP should ideally be in the 7mm Hg to 12mm Hg range.25 The bleb should appear noninflamed, slightly elevated and diffuse with indistinct margins. The bleb walls should be thin and appear microcystic.
Elevated IOP in the early postoperative period may be due to tight suturing of the scleral flap. Tight sutures are often used to avoid postoperative hypotony, with the expectation that they will be cut postoperatively. Suture lysis can be done as early as one week after surgery and as far out as 18 weeks.20
Early postoperative complications include wound leaks, choroidal detachment and bleb infection or failure.20 If a patient has a shallow or flat anterior chamber without a wound leak, clinicians should suspect a choroidal effusion.
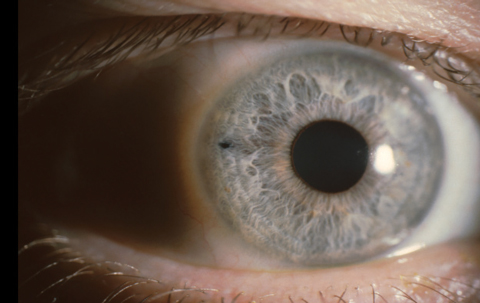 |
| A temporal placement of LPI, above, may decrease the risk of postoperative dysphotopsia. Transillumination, below, is not the best way to test patency. Instead, clinicians should use direct visualization through the iridotomy. |
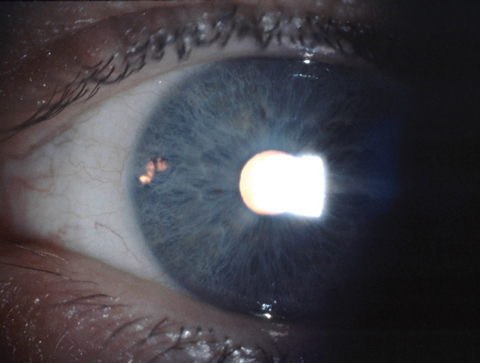 |
Other Procedures for Advanced Glaucoma
Implantation of a glaucoma drainage device and trans-scleral cyclodestructive procedures are also options for advanced glaucoma management. Glaucoma drainage devices, or tube shunts, are often employed in patients with a severely damaged TM, as may occur in neovascular glaucoma or severe uveitis.
Trans-scleral cyclodestructive procedures use a Nd:YAG or diode laser to damage the ciliary body of eyes with refractory glaucoma, impairing the ability to produce aqueous humor. They are generally a last resort in eyes with unsuccessful filtering surgery, eyes with limited vision potential or eyes that are not candidates for other glaucoma procedures.20
Angle-closure Glaucoma
Pupil block is responsible for approximately 90% of all cases of angle-closure glaucoma (ACG).29 In these cases, a laser peripheral iridotomy (LPI) uses an argon or Nd:YAG laser to create a full-thickness hole in the iris to permit aqueous to flow into the anterior chamber without having to pass through the pupil.
When to refer. An LPI should be performed whenever evidence of previous or current angle closure exists.30 Ideally, it is performed prior to the development of acute or chronic IOP elevation and VF loss.
Ancillary anterior chamber imaging can be useful for identifying and quantifying anatomical characteristics that predispose a patient to ACG such as a shallow peripheral or anterior chamber depth, convex iris configuration, reduced anterior chamber volume (typically less than 100mm3), a thicker and anteriorly displaced lens and small corneal diameter.31 These imaging techniques are also useful for assessing the efficacy and patency of an LPI post-surgery.32
Clinicians should perform indentation gonioscopy not only to differentiate appositional closure from synechial closure, but also to gauge the flexibility of the iris. If only minimal pressure is needed to push the iris posteriorly into a concave position, the iris will likely conform to only mild increases in posterior chamber pressure, resulting in iris bombé. An angle that deepens with indentation in which minimal peripheral anterior synechiae (PAS) are present can be expected to do well with LPI.
Clinicians should make every attempt to document some degree of functional or structural damage prior to referring a patient for LPI.30 More sensitive tests such as pattern electroretinography and frequency-doubling VF testing may reveal early damage when conventional OCT and white-on-white VF testing are normal. If any of these tests show signs of early damage in an eye identified as shallow or occludable with gonioscopy, clinicians should strongly consider an LPI.
An LPI may also be performed to prevent ACG in asymptomatic eyes deemed potentially occludable and at substantial risk.30 Although a lack of literature makes it difficult to predict which eyes will go on to develop ACG, most eyes at risk are initially identified with routine Van Herick angle screening. Gonioscopy should be performed on all eyes with a peripheral anterior chamber depth that is ¼ of the peripheral corneal thickness or less (Van Herick grades one and two).29 An eye is considered occludable if the posterior TM is obscured without indentation in two or more quadrants. Such eyes should then undergo a thorough evaluation for structural or functional glaucomatous damage and a pointed history to uncover ACG symptoms. Even in the absence of glaucomatous damage or symptoms, an LPI may be appropriate when multiple ACG risk factors exist or if the patient has limited access to medical care.30 If there is doubt a patient is capable of recognizing the symptoms of acute angle closure and promptly returning to the clinic, clinicians should discuss a prophylactic LPI.
Postoperative care. Patients will normally be prescribed a topical steroid to manage inflammation following LPI surgery. In addition, some patients will be on an IOP-lowering drop. The post-op medications can usually be discontinued at the one-week post-op visit. Some patients will suffer chronic elevation of IOP despite an open anterior chamber angle following resolution of their angle closure and may require long-term treatment.
Complications of LPI are usually mild and transient, including hyphema, anterior uveitis and IOP spike.20 Rare complications include retinal detachment and cataract, and up to 4% of patients may suffer visual disturbances related to light transmission through the iridotomy.33 Iridotomies partially exposed at the upper eyelid margin are most frequently associated with vision disturbance; thus, temporally located LPIs may carry a lower risk of dysphotopsia.34
The patency of the iridotomy should be assessed at each postoperative visit using direct visualization of the lens capsule, zonules or posterior chamber through the iridotomy using the biomicroscope.30 The iridotomy may become occluded by inflammatory debris or clumps of iris tissue in the immediate postoperative period. Because the lens used to create the iridotomy generally offers the best view through it, the surgeon may have to assess patency for difficult cases.
If pupil block was present prior to undergoing LPI, a substantial deepening of the anterior chamber angle is expected following the procedure. The central anterior chamber depth will not change following LPI because the position of the lens is not affected by the procedure. Rather, the chamber volume or angle depth will reflect the deepening that occurs postoperatively.35 Postoperative deepening of the angle will confirm patency of the LPI and that the patient had primary pupil block preoperatively. Shallow anterior chamber angles in the presence of a patent iridotomy may be caused by plateau iris syndrome (PIS), PAS, space-occupying lesions in the posterior chamber or other conditions that produce anterior displacement of the lens-iris diaphragm.30
Other Procedures for ACG
Removal of a cataractous lens will improve the patient’s vision and resolve the pupil block.30 While lens extraction is always curative for pupil block, LPI, as a less-invasive procedure, is the preferred treatment for eyes without cataract. However, a recent study suggests lens extraction could be considered a first-line treatment for ACG even in patients without cataract.36
For patients with ACG without pupil block, LPI is of no benefit. Treatment must be directed toward the cause of the angle closure, such as PIS. PIS is diagnosed when the angle remains predisposed to closure after LPI has been performed. Argon laser peripheral iridoplasty is an effective treatment whereby laser burns placed in the peripheral iris will cause contraction of the iris root and pull the iris out of the angle.37
Many interventions for patients with OAG and ACG can significantly impact long-term outcomes. The trick is knowing when to follow, when to refer and how to care for patients post-procedure. The savvy OD can handle almost any patient with glaucoma, if they incorporate these tips into their glaucoma practice and properly comanage with the surgeon.
Dr. Trevino is an associate professor and director of Residency Programs at the Rosenberg School of Optometry.
Dr. Majcher is an assistant clinical professor at the Rosenberg School of Optometry.
Dr. Sponsel is a professor of Vision Science at the Rosenberg School of Optometry.
| 1. Shaarawy T, Sherwood M, Hitchings R, Crowston J. Glaucoma. 2nd ed. Philadelphia: Saunders; 2014. 2. Kramer T, Noecker R. Comparison of the morphologic changes after selective laser trabeculoplasty and argon laser trabeculoplasy in human eye bank eyes. Ophthalmology. 2001;108(4):733-79. 3. Allingham R, Damji K, Freedman S, et al, eds. Shields’ Textbook of Glaucoma. 6th ed. Philadelphia: Lippencott Williams & Wilkins; 2011. 4. Kennedy J, SooHoo J, Kahook M, Seibold L. Selective laser trabeculoplasty: An update. Asia Pac J Ophthalmol. 2016;5(1):63-9. 5. Latina M, Sibayan S, Shin D, et al. Q-switched 532-nm Nd:YAG laser trabeculoplasty (selective laser trabeculoplasty): a multicenter, pilot, clinical study. Ophthalmology. 1998;105(11):2082-8. 6. Woo D, Healey P, Graham S, Goldberg I. Intraocular pressure-lowering medications and longterm outcomes of selective laser trabeculoplasty. Clin Exper Ophthalmol. 2015;43(4):320-7. 7. Gracner T, Falez M, Gracner B, Pahor D. Long-term follow-up of selective laser trabeculoplasty in primary open-angle glaucoma. Klin Monbl Augenheilkd. 2006;223(9):743-7. 8. Kent S, Hutnik C, Birt C, et al. A randomized clinical trial of selective laser trabeculoplasty versus argon laser trabeculoplasty in patients with pseudoexfoliation. J Glaucoma. 2015;24(5):344-7. 9. Bovell A, Damji K, Hodge W, et al. Long term effects on the lowering of intraocular pressure: selective laser or argon laser trabeculoplasty? Can J Ophthalmol. 2011;46(5):408-13. 10. Weinand F, Althen F. (2006). Long-term clinical results of selective laser trabeculoplasty in the treatment of primary open angle glaucoma. Eur J Ophthalmol. 2006;16(1):100-4. 11. Tojo N, Oka M, Miyakoshi A, et al. Comparison of fluctuations of intraocular pressure before and after selective laser trabeculoplasty in normal-tension glaucoma patients. J Glaucoma. 2014;23(8):138-43. 12. Nagar M, Ogunyomade A, O’Brart D, et al. A randomised, prospective study comparing selective laser trabeculoplasty with latanoprost for the control of intraocular pressure in ocular hypertension and open angle glaucoma. Br J Ophthalmol. 2005;89(11):1413-7. 13. Melamed S, Ben Simon G, Levkovitch-Verbin H. Selective laser trabeculoplasty as primary treatment for open-angle glaucoma: a prospective, nonrandomized pilot study. Arch Ophthalmol. 2003;121(7):957-60. 14. McIlraith I, Strasfeld M, Colev G, Hutnik C. Selective laser trabeculoplasty as initial and adjunctive treatment for open-angle glaucoma. J Glaucoma. 2006;15(2):124-30. 15. Giaconi J, Law S, Nouri-Mahdavi K, et al, eds. Pearls of Glaucoma Management. 2nd ed. Berlin: Springer-Verlang; 2016. 16. Martow E, Hutnik C, Mao A. SLT and adjunctive medical therapy: a prediction rule analysis. J Glaucoma. 2011;20(4):266-70. 17. Wasyluk J, Piekarniak-Wozniak A, Grabska-Liberek I. The hypotensive effect of selective laser trabeculoplasty depending on iridocorneal angle pigmentation in primary open angle glaucoma patients. Arch Med Sci. 2014;10(2):306-8. 18. Samples J, Schacknow P, eds. Clinical Glaucoma Care: The essentials. New York: Springer; 2014. 19. Song J. Complications of selective laser trabeculoplasty: A review. Clin Ophthalmol. 2016;10:137-43. 20. Kahook M, ed. Essentials of Glaucoma Surgery. Thorofare, NJ: Slack Inc.; 2012. 21. Ellex iScience. Ab-interno canaloplasty - The minimally invasive glaucoma surgery that keeps its promise: 12-month case series review. 2016. www.ellex.com/wp-content/uploads/sites/9/Ellex-ABiC-Whitepaper-12-Months-ELECTRONIC.pdf. Accessed May 18, 2017. 22. Seibold L, SooHoo J, Kahook M. Endoscopic cyclophotocoagulation. Middle East Afr J Ophthalmol. 2015;22(1):18-24. 23. Rathi S, Radcliffe N. Combined endocyclophotocoagulation and phacoemulsification in the management of moderate glaucoma. Surv Ophthalmol. March 2, 2017. [Epub ahead of print]. 24. Chen P, Lin S, Junk A, et al. The effect of phacoemulsification on intraocular pressure in glaucoma patients: a report by the American Academy of Ophthalmology. Ophthalmology. 2015;122(7):1294–1307. 25. Kahook M, Schuman J, eds. Chandler and Grant’s Glaucoma. Thorofare, NJ: Slack Inc.; 2013. 26. Drance S. The glaucomatous visual field. Invest Ophthalmol. 1972;11(2):85-96. 27. Peters D, Bengtsson B, Heijl A. Threat to fixation at diagnosis and lifetime risk of visual impairment in open-angle glaucoma. Ophthalmology. 2015;122(5):1034-9. 28. Fechtner R. Maximal medical therapy. J Glaucoma. 2001;10 (5):S73-5. 29. Wormington C. Ophthalmic Lasers. Oxford: Butterworth-Heinemann; 2003. 30. Prum B, Herndon L, Moroi S, et al. Primary angle closure preferred practice pattern guidelines. Ophthalmology. 2016;123(2):P1-P40. 31. Guo X, He M. Angle-closure glaucoma: Risk factors. In: Giaconi J, Law S, Nouri-Mahdavi K, et al, eds. Pearls of Glaucoma Management. 2nd ed. Berlin: Springer-Verlang; 2016. 32. Li S, Wang H, Mu D, et al. Prospective evaluation of changes in anterior segment morphology after laser iridotomy in Chinese eyes by rotating Scheimpflug camera imaging. Clin Exp Ophthalmol. 2010;38(1):10-4. 33. Spaeth G, Idowu O, Seligsohn A, et al. The effects of iridotomy size and position on symptoms following laser peripheral iridotomy. J Glaucoma. 2005;14(5):364-7. 34. Vera V, Naqi A, Belovay G, et al. Dysphotopsia after temporal versus superior laser peripheral iridotomy: a prospective randomized paired eye trial. Am J Ophthalmol. 2014;157(35):929-35. 35. Lee K, Sung K, Shon K, et al. Longitudinal changes in anterior segment parameters after laser peripheral iridotomy assessed by anterior segment optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(5):3166-70. 36. Azuara-Blanco A, Burr J, Ramsey C, et al. Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): a randomised controlled trial. Lancet. 2016;388(10052):1389-97. 37. Ramakrishnan R, Mitra A, Abdul Kader M, Das S. To study the efficacy of laser peripheral iridoplasty in the treatment of eyes with primary angle closure and plateau iris syndrome, unresponsive to laser peripheral iridotomy, using anterior-segment oct as a tool. J Glaucoma. 2016;25(5):440-6. 38. Sponsel WE, Groth SL. Mitomycin-augmented non-penetrating deep sclerectomy: preoperative gonioscopy and postoperative perimetric, tonometric and medication trends. Br J Ophthalmol. 2013;97:357-61. |

