Seeing Systemic Disease within the EyeThis month in Review of Optometry, multiple ODs explain the connection between the eye and various systemic conditions, including hypertension, stroke, sleep disorders, COVID-19, diabetes, thyroid eye disease and more. Check out the other articles featured in the October issue:
|
Diabetes, as described by the Centers for Disease Control (CDC), is a chronic condition that affects how your body turns food into energy. It’s mainly characterized by elevated blood sugar. The disease occurs when the body does not make any insulin (type 1) or is unable to properly use insulin or produce sufficient insulin (type 2). As a result, blood sugar (glucose) builds up in the blood, giving rise to several complications within the body including of the eyes, kidneys and limbs.1
The National Diabetes Statistic Report from the CDC reports that a total of 37.3 million people have diabetes, which is roughly 11.3% of the US population.2 The condition can lead to several additional medical problems including high blood pressure, high cholesterol, heart disease, stroke, kidney failure, leg and foot amputation and early death.1 According to the CDC, diabetes is the leading cause of new cases of blindness among adults 18 to 64 years old. Data from 2019 revealed that among US adults 18 and older diagnosed with diabetes, 11.8% reported severe vision difficulty or blindness.2
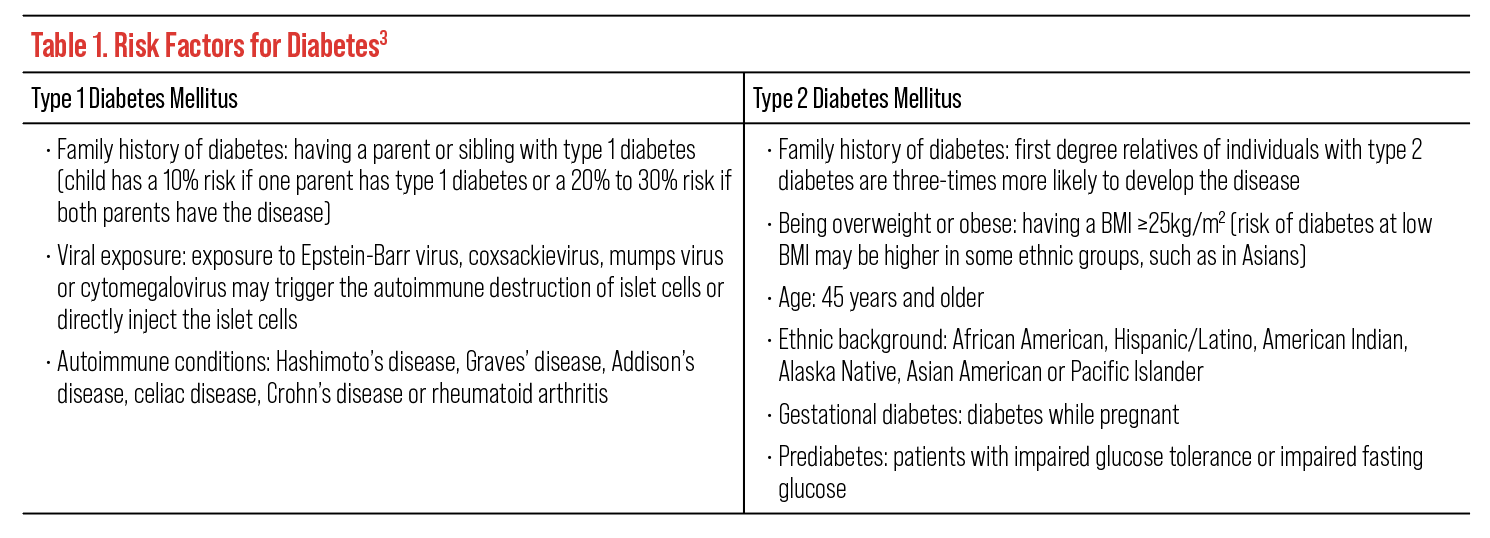
When evaluating a patient with diabetes, there are two critical factors to remember for proper management. First, it is important to obtain the patient’s full history of diabetes, as it can contribute to their risk of disease complications. Important risk factors to consider and ask patients about include family history of diabetes, history of viral exposure that may have triggered autoimmune destruction of islet cells (for type 1, examples include Epstein-Barr and cytomegalovirus), autoimmune disease, BMI, age, ethnic background, gestational diabetes, prediabetes, hypertension, cholesterol levels and amount of physical activity (Table 1).3
Patients with longer durations of diabetes, higher levels of glycemia, greater BMI, higher blood pressure and presence of nephropathy are at greater risk of vision loss from diabetic ocular complications.4
Second, it is important to familiarize yourself with the ocular manifestations from diabetes that can be detected early in the disease process to help prevent severe complications, most notably blindness.
 |
| Click image to enlarge. |
Surveillance for diabetic retinopathy is an integral part of eye exams in patients with diabetes largely due to it being one of the most common causes of severe visual impairment. Diabetic retinopathy can be classified broadly as nonproliferative and proliferative retinopathy. Nonproliferative diabetic retinopathy is classified into categories depending on prognostic value of lesion severity and may show microaneurysms, hemorrhages, lipids and/or soft exudates.5 This stage of the disease occurs early, and patients are most often asymptomatic in the absence of significant macular edema. Progression to proliferative diabetic retinopathy involves advanced damage to the retinal capillaries resulting in the development of hypoxia, ischemia and neovascularization that could lead to vitreous hemorrhage and/or tractional retinal detachment and consequent severe vision loss.5
Diabetic macular edema can occur at any stage of retinopathy and is the most common cause of vision loss in those with diabetic retinopathy.6 Macular edema results from a buildup of intraretinal fluid within the macula and may present with or without the presence of lipid exudates.3
While diabetic retinopathy and/or diabetic macular edema may be the most common manifestation of diabetes in the eye, it is important to remember that all ocular structures can be affected by diabetes and can be evident without the presence of diabetic retinopathy.
Diagnosing these nonretinal ocular complications can also help with early diagnosis and management of diabetes to prevent sight- and life-threatening results.
There are seven nonretinal ocular diabetes complications to remember when evaluating a patient with diabetes: prescription and vision changes, cranial nerve palsies, dry eye, neurotrophic cornea, cataracts, vitreous degeneration or detachment and optic neuropathies (Figure 1).
In this article, we’re going to discuss the signs, symptoms, risk factors and potential management and treatment options of each nonretinal complication to look out for and consider when evaluating patients with diabetes.
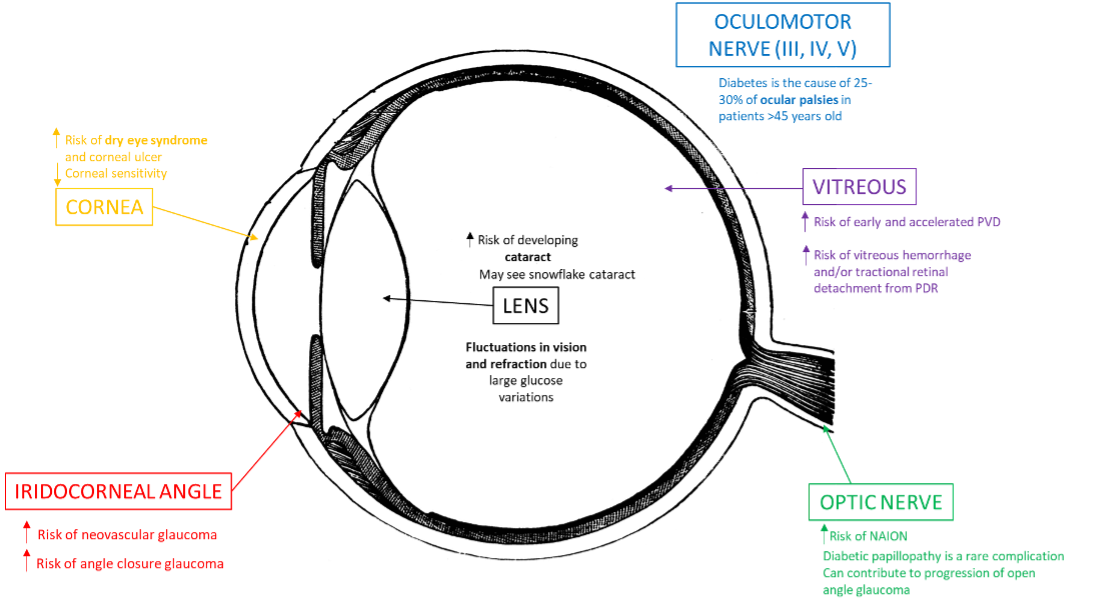 |
| Fig. 1. Shown here are several nonretinal ocular complications that may present in patients with diabetes.3,7 Click image to enlarge. |
1. Prescription/Vision Changes
One of the first measurements we perform in an eye exam involves the assessment of the patient’s visual acuity and refractive status; consequently, detected or patient-reported changes in vision are often the first signs or symptoms of undiagnosed or uncontrolled diabetes.
More specifically, transient changes or fluctuations in vision and prescription can be a key sign of impaired glucose control and may also occur after introduction of diabetes treatment with rapid improvement of glucose control.7
These fluctuations can be hyperopic or myopic due to excess glucose entering via the aqueous humor, which then leads to excess fluid absorption of glucose by the crystalline lens.7 A myopic shift occurs from lens swelling due to the activation of the aldose reductase pathway inducing the intracellular accumulation of sorbitol.7 On the other hand, a hyperopic shift occurs when there is a significant reduction of the concentration of glucose in the aqueous humor.7 It is also important to note that a hyperopic shift may also happen with the development of diabetic macular edema from artificially reducing axial length or from cortical cataract formation that is more common in diabetes.7 These refractive shifts can be several diopters or more; therefore, it is not recommended to finalize the prescription until these large glycemic variations are normalized and stable.
Patients with poorly controlled or long-term diabetes may also present with acquired color vision changes including blue-yellow and/or red-green deficiencies.3 The development of color vision changes can precede the development of diabetic retinopathy or present in patients who have already been diagnosed with the condition and/or macular edema.3 The degree of color vision impairment increases with the severity of the macular edema.3,7
 |
|
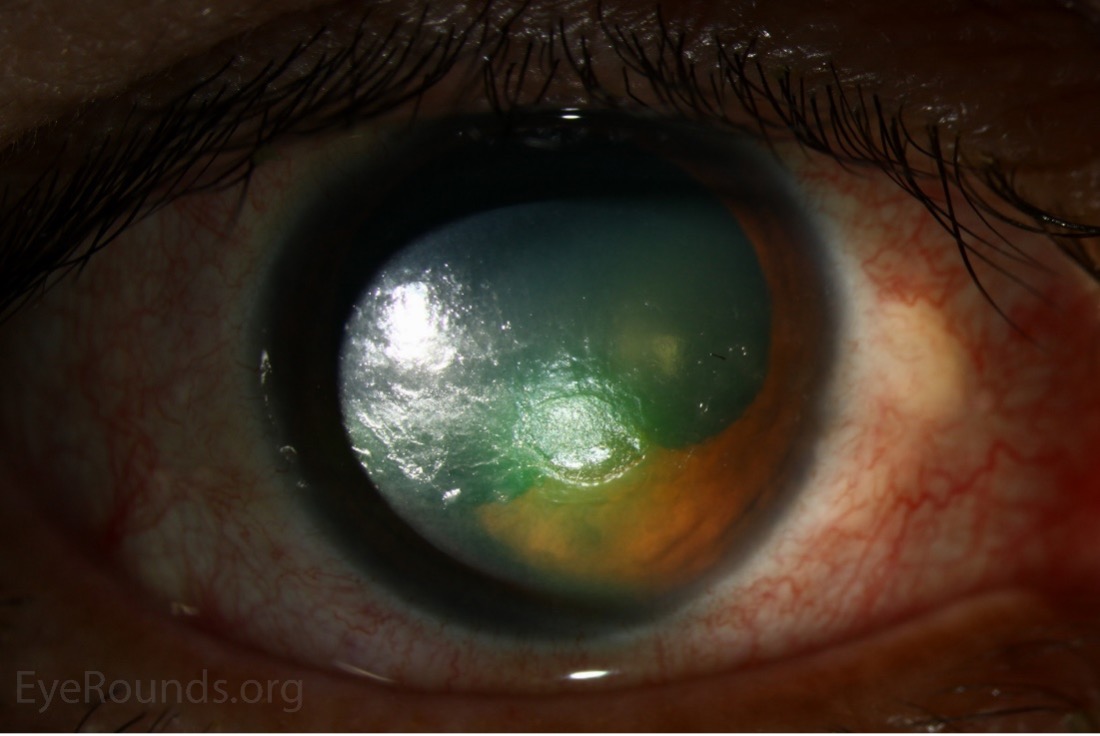
Fig. 2. This image shows a patient with a neurotropic corneal ulcer, a finding that may present in some patients with poorly controlled diabetes. Photo: Brice Critser, CRA. Click image to enlarge. |
Additionally, altered accommodative dysfunction, visual field changes and decreased contrast sensitivity may present in patients with uncontrolled blood sugar or with a history of diabetic retinal disease and treatment with panretinal laser photocoagulation.3
When patients present with vision fluctuations and changes because of hyperglycemia, it is important to fully assess the eye with a dilated exam to rule out retinal involvement and refer to a retinal specialist for treatment in cases involving more advanced disease. Educating patients about inconsistent refractive status due to uncontrolled blood sugar is important to encourage stability. A follow-up exam for a more accurate refraction is recommended after glucose control has been stabilized for at least several weeks.7
2. Cranial Nerve Palsies
Ocular motility disorders can develop secondary to diabetic neuropathies involving the third, fourth or sixth cranial nerves often with binocular diplopia but pupillary-sparing signs and symptoms.7 Diabetes is the cause of 25% to 30% of ocular palsies in patients older than 45 with isolated sixth nerve palsy being five-times more frequent in patients with diabetes compared with nondiabetic patients.7 Third nerve palsies are also among the more common diabetes-related neuropathies with patients presenting with signs of eyelid ptosis, exotropia and hypotropia of the affected eye with or without acute pain.3
Here are some notes on the variations between third, fourth and sixth nerve palsies:
- Usually, third nerve palsies associated with diabetes do not involve the pupil, which is an important diagnostic feature to help rule out other neurological disorders, including compressive lesions or intracranial aneurysms.3 This can also be confirmed with an MRI of the head.
- Patients with fourth nerve palsies present with complaints of sudden-onset vertical diplopia with the deviation worsening with downward or lateral gaze away from the affected muscle and with the head tilted to the side of the affected muscle.3
- Patients with sixth nerve palsies may complain of horizontal diplopia with the affected eye being esotropic.3 Patients may also turn their heads in the direction of paralysis to reduce the diplopia.3
Recovery from these ocular motility palsies can take two to six months without sequalae with the management of other systemic and vascular risk factors including blood sugar and pressure control; however, recurrences are common, especially with recurrent hyperglycemia.3,7
Although most diabetic neuropathies are pupil sparing, diabetes can affect the sympathetic innervation of the iris resulting in sluggish pupillary reflexes.3 Pupils therefore may be more miotic and have a weaker reaction to topical mydriatic drops.3 Furthermore, patients with a history of panretinal photocoagulation may have an increased pupillary size due to potential damage to the short and long ciliary nerves.3
Careful assessment of the pupils and ocular motilities in multiple positions of gaze is crucial when evaluating cranial palsies. After ruling out other life-threatening and neurological etiologies, counseling patients about the importance of blood sugar control and the length of time for resolution is recommended. Providing an eye patch for temporary relief of diplopia may also be warranted.
3. Dry Eye
Symptoms of dry eye are a common reason why patients seek eye care, and the condition has been linked to diabetes. Patients often present to the clinic with symptoms of ocular discomfort, irritation, grittiness or foreign body sensation with accompanying fluctuating vision changes. However, some patients with diabetes may not have any symptoms due to corneal sensory neuropathy; thus, an evaluation of the cornea for epithelial defects is important.3
Studies have shown a higher prevalence of dry eye syndrome resulting from tear film abnormalities in patients with diabetes compared with the general population.7 One study found that 53% of people with diabetes reported symptoms of dry eye compared with 9% of individuals without diabetes.5 Common dry eye findings in patients with diabetes include meibomian gland dysfunction and reduced corneal sensitivity. The latter is due to the neuropathy of the ophthalmic division of the trigeminal nerve leading to reduced reflex tear secretion and increased risk of neurotrophic keratitis.3 Damage to the microvascular supply to the lacrimal gland can also occur in patients with a long history of diabetes thereby impairing lacrimation.3 The reduced tear production and impaired corneal sensitivity delays corneal healing, so patients should be closely monitored to assess for infection.5
Management of patients with dry eye includes the use of lubricating eye drops and proper eyelid disease treatment. Close monitoring of patients with abrasions, recurrent corneal erosion, nonspecific keratitis (NK) and/or corneal ulceration is important, and treatment with the use of bandage contact lens or patching, sodium chloride solutions/ointments, corticosteroids, antibiotic drops or other types of ocular surface treatment may be warranted.3,5 Educating patients on the importance of diabetes control will also aid in the management of dry eye disease (are you detecting a pattern yet with that advice?).
4. Neurotrophic Keratitis
As mentioned, patients with diabetes may have decreased corneal sensation from neuropathy of the ophthalmic division of the trigeminal nerve, putting them at risk for NK.3 There are also structural changes that occur within the cornea that can predispose patients with diabetes to NK. Chronic hyperglycemia results in a thickened basement membrane, resulting in poor adhesion between the basement membrane and the stroma.8,9 This poor adhesion along with delayed wound healing can make neurotrophic corneal ulcers difficult to manage in patients with diabetes (Figure 2).
NK should be suspected in patients who have epithelial defects without significant symptoms. Corneal nerve sensitivity testing with a cotton swab wisp, dental floss or corneal esthesiometer should be performed to confirm the diagnosis, and four corneal quadrants should be tested and compared between the eyes. Early NK may look like epithelial staining, which can be managed with copious preservative-free artificial tears and lubricating ointment at night. If there is a large epithelial defect without stromal involvement, a bandage contact lens should be placed on the eye, and prophylactic antibiotic eyedrops should be added. The addition of an amniotic membrane or autologous serum can be used for persistent cases.
If the epithelial defect progresses to an ulcer with stromal involvement, the recommended treatment is tarsorrhaphy with the addition of oral tetracyclines and vitamin C. Corneal neurotization can be performed on patients with recurrent or nonhealing corneal ulcers. Corneal neurotization is the process of grafting healthy nerve tissue onto the distal portion of the damaged corneal nerve to re-establish the neuronal pathway.10
5. Cataracts
 |
|
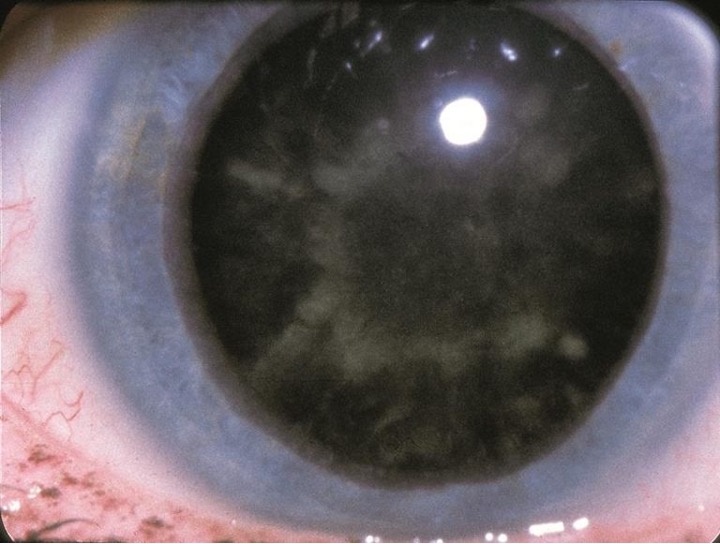
Fig. 3. This patient has what’s been coined a “snowflake cataract,” a rare complication mainly seen in patients with poorly controlled type 1 diabetes. Photo: American Academy of Ophthalmology. Click image to enlarge. |
Patients with type 2 diabetes are four-times more likely to have cataracts than patients without diabetes.11 It’s thought that cataracts progress faster in patients with diabetes due to an increased deposition of advanced glycation end products.12 The incidence of cataract formation and progression also increases with increased duration of diabetes and poorer blood glucose control.12 There is an association with increased incidence of posterior subcapsular cataracts, nuclear sclerotic cataracts and cortical cataracts in patients with diabetes.12
A rare type of cataract that can occur in uncontrolled diabetes is the snowflake cataract (Figure 3). These are generally seen in patients with type 1 diabetes who have poor blood sugar control. A snowflake cataract is described as anterior and posterior cortical opacities with a snowflake-like pattern.13 There have been case reports describing the reversal of the cataract with improved blood sugar control.13
The mainstay treatment for cataracts in patients with diabetes is cataract surgery. It’s important to note that cataract surgery may cause diabetic retinopathy or diabetic macular edema to progress.11 Consider the implications cataract surgery may have on your patient’s visual outcome before referring them. It may be prudent to wait until more severe diabetic retinopathy or any macular edema is appropriately managed by retina providers before thinking about a surgical evaluation of cataract.
6. Vitreous
Elevated blood glucose levels can affect the structural components of the vitreous.14 Studies have detected non enzymatic glycation of the vitreous collagen in diabetic patients from elevated vitreous glucose levels.14,15 This can then lead to early and accelerated vitreous degeneration or partial posterior vitreous detachments.14 Partial posterior vitreous detachments can play a role in proliferative diabetic retinopathy. As new blood vessel growth on the retina expands into the posterior vitreous cortex, it can increase the incidence of vitreous hemorrhage and/or tractional retinal detachment from vitreous traction on these new vessels.3
7. Optic Neuropathies
Patients with diabetes are at increased risk for the following forms of optic neuropathy:
 |
|
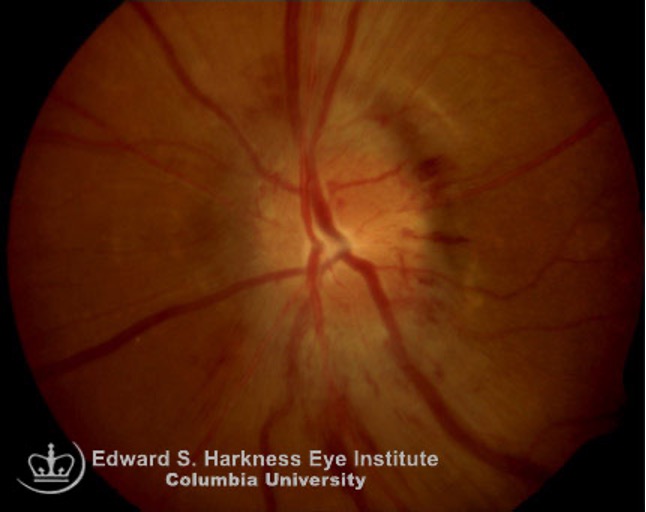
Fig. 4. Diabetic papillopathy, pictured here, is an atypical form of NAION causing no physical symptoms. Photo: Columbia Eye. Click image to enlarge. |
Diabetic papillopathy. This is a rare complication of diabetes with an incidence rate of 0.5% (Figure 4).11 The pathogenesis of diabetic papillopathy is not fully understood but thought to be a consequence of limited vascular supply to the peripapillary area and is associated with rapid reductions in blood glucose level. 9
Patients with diabetic papillopathy generally present with mildly reduced visual acuity. They will have unilateral or bilateral hyperemic nerve swelling and may not present with an afferent pupillary defect (APD) or dyschromatopsia. Typically, they will present with some form of diabetic retinopathy, but not always. The visual field may only show an enlarged blind spot without any other defects.
Diabetic papillopathy is a diagnosis of exclusion. Head imaging and labs should be ordered to rule out other, more ominous forms of optic neuropathy. Diabetic papillopathy tends to resolve within a few months to a year, and the vision will go back to normal or near-normal. There is no current treatment for diabetic papillopathy other than blood sugar control.
Non-arteritic anterior ischemic optic neuropathy (NAION). This occurs when microvascular changes lead to ischemia of the anterior portion of the optic nerve. Although diabetes is not a direct cause of NAION, it can increase a patient’s risk of developing the condition.16 One study showed that up to 25% of patients with NAION also have diabetes.12
NAION presents as unilateral vision loss with a swollen optic nerve. There is typically an APD present, and visual field testing may show an altitudinal defect. The contralateral eye will show a “disc at risk” with a small cup-to-disc ratio. Immediate testing for ESR, CRP and platelet count must be ordered to rule out giant cell arteritis, especially in patients older than 55.
There is currently no treatment for NAION. It’s important to stress the benefits of blood pressure, blood sugar and cholesterol control to these patients. They should also discuss with their primary care physician about potentially avoiding blood pressure medications at night to prevent nocturnal hypotension, which may be a predisposing factor. However, you will need to weigh the pros and cons in this situation, as taking blood pressure medications in the morning rather than the evening has been shown to increase the risk of stroke and heart-related death.
Finally, patients should be counseled on the risk of occurrence to the contralateral eye.
Glaucoma. There is conflicting evidence showing diabetes is a risk factor for glaucoma and it’s thought that diabetes can contribute to the progression of open-angle glaucoma. One study showed that open-angle glaucoma patients with diabetes have lower retrobulbar flow in the central retinal artery, suggesting that diabetes influences the vasculature of the optic nerve and can therefore contribute to disease progression.17 The study also found that open-angle glaucoma patients with diabetes appear to have more impairment of vascular regulation compared with open-angle glaucoma patients without diabetes.17
With this in mind, you may want to consider targeting a lower intraocular pressure in your patients with diabetes to further reduce the stress on the optic nerve and help prevent glaucoma progression.
Patients with diabetes are at higher risk for other types of glaucoma as well. Neovascular glaucoma is a well-known complication of proliferative diabetic retinopathy that can lead to angle closure.7 This patient population may also be at increased risk for angle-closure glaucoma due to increased prevalence and progression of cataract formation.7
Summary
Although retinopathy is a significant part of diabetic eye disease, it is important to remember that diabetes can affect all portions of the eye. Being familiar with these complications can help with early diagnosis and management of the disease to prevent further ocular or vision damage. Take the time to evaluate your patients’ eyes from front to back.
Remember that most of the issues related to diabetic eye disease can be managed with consistently improved glucose control from the time of diagnosis. Educate your patients about your exam findings and how they relate to diabetes. Encourage them to regulate their blood sugar not only through prescribed medications, but also through lifestyle modifications, mainly diet and exercise. Lastly, be sure to congratulate your patients who have achieved better blood sugar control, as this is not an easy feat!
Dr. Loo is a staff optometrist and eye clinic section chief at the Kernersville VA Health Care Center. She graduated from the Illinois College of Optometry and completed her residency in primary care/ocular disease at the Salisbury VA Medical Center. Dr. Werner is a staff optometrist at the Kernersville VA Health Care Center. She graduated from Southern College of Optometry and completed her residency in primary care/low vision rehabilitation at the VA Boston Healthcare System. They have no financial interests to disclose.
1. Diabetes report card 2021. CDC. https://www.cdc.gov/diabetes/library/reports/reportcard.html. Accessed July 2022. 2. National Diabetes Statistics Report: Coexisting Conditions and Complications. CDC. https://www.cdc.gov/diabetes/data/statistics-report/coexisting-conditions-complications.html. Accessed July 2022. 3. Evidence-based clinical practice guideline: eye care of the patient with diabetes mellitus. second edition. American Optometric Association. Published October 2019. https://aoa.uberflip.com/i/1183026-evidence-based-clinical-practice-guideline-eye-care-of-the-patient-with-diabetes-mellitus-second-edition/3?m4=. Accessed July 2022. 4. Klein R, Klein BEK. Epidemiology of ocular functions and diseases in persons with diabetes. In: Cowie CC, Casagrande SS, Menke A, et al. Diabetes in America. 3rd ed. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases (US). 2018. 5. Seewoodhary M. An overview of diabetic retinopathy and other ocular complications of diabetes mellitus. Nurs Stand. 2021;36(7):71-76. 6. Browning DJ, Stewart MW, Lee C. Diabetic macular edema: Evidence-based management. Indian J Ophthalmol. 2018;66(12):1736-50. 7. Feldman-Billard S, Dupas B. Eye disorders other than diabetic retinopathy in patients with diabetes. Diabetes Metab. 2021;47(6):101279. 8. Cousen P, Cackett P, Bennett H, Swa K, Dhillon B. Tear production and corneal sensitivity in diabetes. Journal of Diabetes and its Complications. 2007;21(6):371-73. 9. Sayin N, Kara N, Pekel G. Ocular complications of diabetes mellitus. World Journal of Diabetes. 2015;6(1):92-108. 10. American Academy of Ophthalmology. Corneal neurotization for neurotrophic keratitis. June 27, 2022. EyeWiki. https://eyewiki.aao.org/Corneal_Neurotization_for_Neurotrophic_Keratitis. Accessed August 2, 2022. 11. Skarbez K, Priestley Y, Hoepf M, Koevary SB. Comprehensive Review of the Effects of Diabetes on Ocular Health. Expert Rev Ophthalmol. 2010;5(4):557-77. 12. Jeganathan VS, Wang JJ, Wong TY. Ocular associations of diabetes other than diabetic retinopathy. Diabetes Care. 2008;31(9):1905-12. 13. Jin YY, Huang K, Zou CC, Liang L, Wang XM, Jin J. Reversible cataract as the presenting sign of diabetes mellitus: Report of two cases and literature review. Iran J Pediatr. 2012;22(1):125-28. 14. Sebag J, Ansari RR, Dunker S, Suh KI. Dynamic light scattering of diabetic vitreopathy. Diabetes Technol Ther. 1999;1(2):169-76. 15. Sebag J. Diabetic vitreopathy. Ophthalmol. 1996;103(2):205-6. 16. Lee MS, Grossman D, Arnold AC, Sloan FA. Incidence of nonarteritic anterior ischemic optic neuropathy: increased risk among diabetic patients. Ophthalmol. 2011;118(5):959-63. 17. Shoshani Y, Harris A, Shoja MM, et al. Impaired ocular blood flow regulation in patients with open-angle glaucoma and diabetes. Clinical & Experimental Ophthalmol. 2012;40(7):697-705. |