Retina on the RiseExperts offer pearls for detecting and managing numerous posterior segment conditions in the June 2023 issue of Review of Optometry, our 14th annual Retina Report. Featured articles cover everything from AMD staging, referral timing, interpreting vitreous opacities and using nutrition to promote retinal health. Check out the other articles featured in this issue:
|
Age-related macular degeneration (AMD) is a common condition that can cause significant central loss of vision. According to the CDC, in 2019, an estimated 19.8 million Americans aged 40 years and older were affected by some degree of AMD.1 Its prevalence also increases with age, from 2% among people aged 40 to 44 to 46.6% among people aged 85 and older.1
AMD is a specific retinal degenerative disease with distinct clinical features primarily associated with alteration of Bruch’s membrane/choroidal complex, retinal pigment epithelium (RPE) and photoreceptor cells in the macula which is defined as the central 5mm of the retina.2 Aside from aging, genetic susceptibility, cigarette smoking, obesity and higher BMI, female gender, white race, cardiovascular disease, hypertension, hypercholesteremia and poor dietary habits are also among the risk factors for AMD.3-6
Several challenges remain in the detection and management of AMD. Approximately 25% of cases go undetected during routine eye examinations.7 In addition, there are several macular degenerative and non-degenerative conditions with phenotypic and genotypic similarities to AMD that can be mistaken for and misdiagnosed as AMD. Two common ones include adult vitelliform macular dystrophy and Stargardt’s disease.8
Here, we’ll discuss how to interpret clinical findings and use multimodal imaging to more accurately diagnose and classify AMD. We will also walk through three case examples of patients misdiagnosed with AMD presenting with classic signs of other forms of macular degenerative disease. To wrap up, we’ll review the current and future treatment and management options for patients with varying levels of AMD.
AMD Risk Factors3-6In no particular order, the following factors have been linked to an increased risk of AMD:
|
Case 1
A 70-year-old Caucasian female presents for second opinion. She has had reduced vision for the last four years that has worsened in the past two years, particularly in her left eye. She has never smoked. Her medical history was remarkable for systemic hypertension for six years. Her ocular history was remarkable for cataract surgery and YAG capsulotomy nearly 10 years ago. She was diagnosed with AMD by more than one eyecare provider and never received an explanation for the severe vision loss in her left eye.
The patient’s best-corrected visual acuity (BCVA) was 20/40 OD and 20/200 OS. All other findings were unremarkable except for posterior chamber intraocular lens and YAG OU. Her fundus examination was remarkable for a large central vitelliform lesion OD and a circular foveal atrophic area OS (Figure 1A/B). Her optical coherence tomography (OCT) was remarkable for a subretinal hyperreflective lesion in the foveal area, as well as a few drusen and drusenoid pigment epithelial detachments (PEDs) in the right eye and a central area of outer retinal atrophy with extrafoveal drusen in the left (Figure 1C/D).
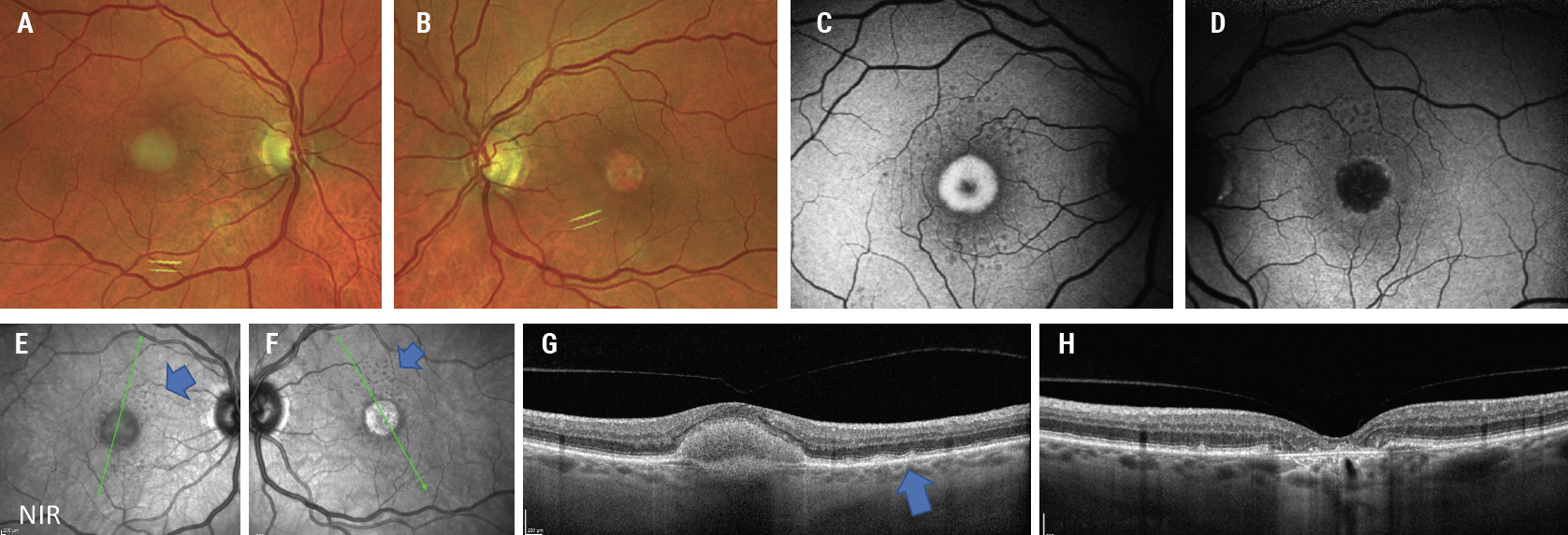 |
| Fig. 1. Fundus photos show a classic foveal vitelliform lesion OD (A) and an atrophic foveal lesion OS (B). FAF of the right eye (C) shows hyperautofluorescence associated with increased lipofuscin, and the left eye (D) shows hypoautofluorescence associated with loss of RPE. The near-infrared reflectance en face images (E, F) show drusen and central lesions (blue arrows). OCT of the right eye (G) shows the central hyperreflective PED lesion and few drusen (blue arrow), and the left eye OCT (H) shows the central RPE and outer retinal atrophy. Click image to enlarge. |
Drusen surrounding the central lesions is also appreciable on en face infrared reflectance of the patient’s OCT (Figure 1E/F). Fundus autofluorescence (FAF) shows a central hyperautofluorescent lesion OD with a central hypoautofluorescent lesion OS and multiple small hypoautofluorescent lesions OU surrounding the central lesions (Figure 1G/H).
These findings are the dominant reason for the patient’s visual loss due to adult-onset vitelliform dystrophy, an autosomal dominant macular degenerative disease often resulting in central vision loss. While the right eye shows a classic vitelliform or “egg yolk” lesion, the left eye shows the sequela following the dissolution of the vitelliform lesion it results in atrophy.9 The presence of drusen could suggest a concurrence of AMD.
With no effective treatment for adult vitelliform currently available, this patient will require periodic monitoring—the same as those with dry AMD—due to possibility of choroidal neovascularization.10
 |
|
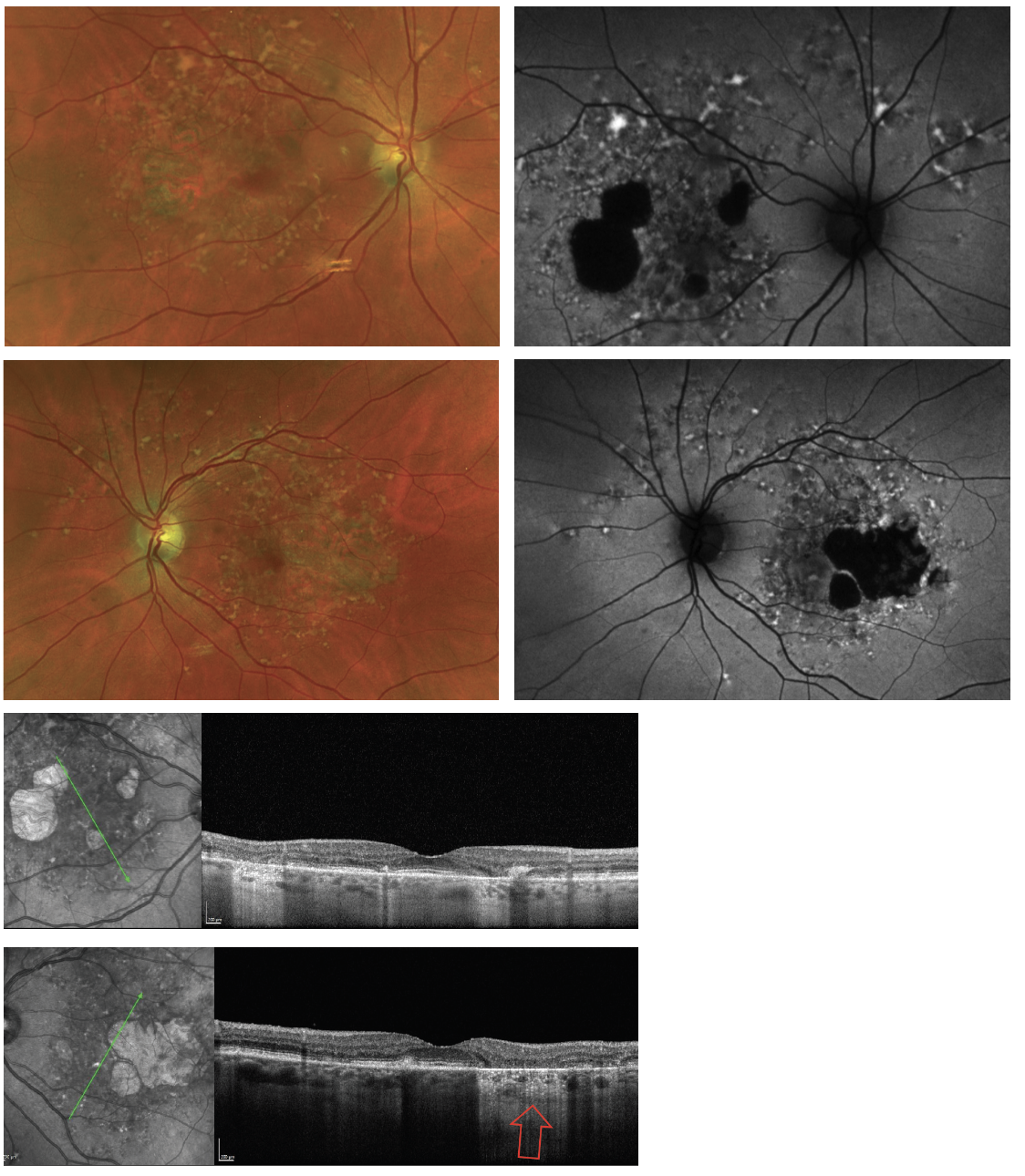
Fig. 2. The yellowish-flecked lesions shown above are pisiform and pisciform lesions. They can be visualized on fundus photos and more notably on FAF as hyperautofluorescent lesions due to accumulation and increased lipofuscin. They can also be seen as atrophic lesions noted as hypoautoflurescence on FAF and by increased hypertransmission on OCT (red arrow). These are all signs of advanced Stargardt’s disease. Click image to enlarge. |
Case 2
A 78-year-old Caucasian female presents for evaluation of AMD with possible geographic atrophy (GA). The patient complains of some degree of “blurred vision” but reports that it has not caused significant difficulties in her daily life. She is a nonsmoker and has had systemic hypertension for more than 10 years. She had cataract surgery several years earlier and is aware of her diagnosis of AMD. Her BCVA is 20/20 in both eyes. Anterior segment examination is remarkable for posterior chamber intraocular lens OU.
Fundus examination revealed several yellowish flecked lesions (pisiform and pisciform) throughout the posterior pole with additional large areas of outer retinal atrophy were present temporal to the fovea (Figure 2). The view of the lesions was enhanced by FAF imaging. OCT showed areas of incomplete and complete RPE and outer retinal atrophy as well as thickening and increased lipofuscin within the RPE. These findings are consistent with advanced Stargardt’s disease, an autosomal recessive disease caused by the alteration of the ABCA4 gene, which is also associated with other macular degenerative diseases including AMD.11
This patient had been seen at our clinic 14 years earlier and was noted to have yellowish flecked lesions (pisciform lesions) on clinical examination and FAF, as well as alteration of the RPE and outer retina (Figure 3). At that time, she was diagnosed with Stargardt disease based on the presence of classic findings. This case, just like the first one, warrants periodic surveillance. Novel therapies such as use of complement inhibitors for Stargardt’s patients are currently under investigation and will eventually allow us to intervene in the disease more aggressively.12
 |
|
Fig. 3. Same patient as in Figure 2, 14 years younger showing an earlier stage of Stargardt disease. Classic pisciform lesions are seen on fundus photos and FAF. At this stage, the right eye of the patient had already begun with RPE atrophy, while the left eye only demonstrated areas of increased localized deposits of lipofuscin. Click image to enlarge. |
Classifying the Disease
Proper staging of AMD relies on a thorough review of the clinical data and images. The presence of small yellowish deposits known as drupelets and drusen between the RPE and Bruch’s membrane is the earliest sign of AMD. Although these deposits can present due to natural aging, detection during clinical examination should raise the eyecare provider’s suspicion (Figure 4).13 Additionally, risk factors previously mentioned and subjective and/or objective findings such as genetic testing, reduced dark adaptation and other clinical findings such as reduced choroidal thickness detectable on SD-OCT are other clues in the differential diagnosis of AMD vs. normal aging.14-15
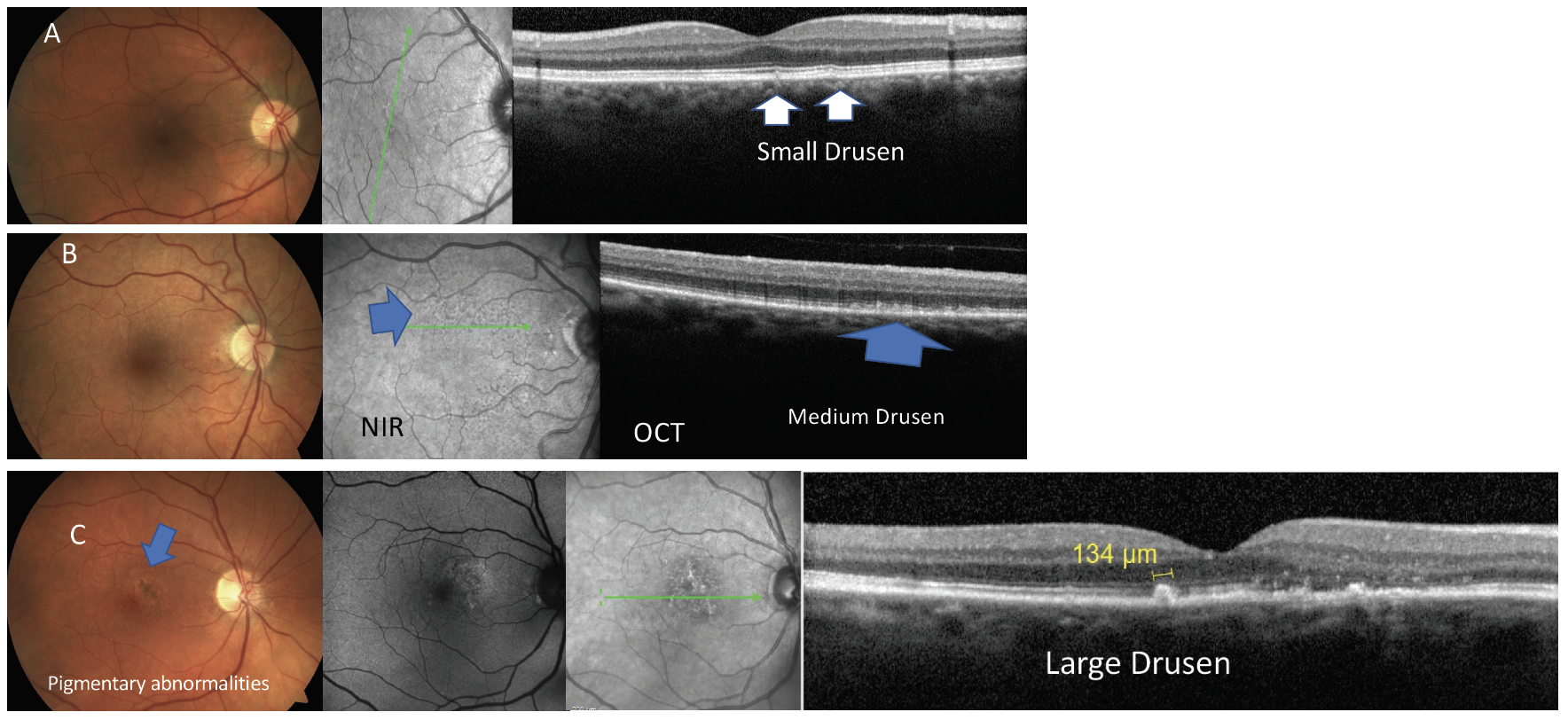 |
Fig. 4. Examples of the stages of dry or atrophic macular degeneration shown by common imaging modalities. Click image to enlarge. |
Table 1. Beckman AMD Classification11 | |
Classification of AMD | Clinical Findings |
Normal Aging | Drupelets or small drusen <63µm |
Early AMD | No pigmentary abnormalities Medium drusen >63µm to <125µm |
Intermediate AMD | Pigmentary abnormalities and/or large drusen |
The basic classification of AMD is considered in a person aged 55 or older, with clinical findings within a two-disc diameter of the fovea.13 According the Beckman classification (Table 1), early AMD is defined as presence of medium size drusen (>63µm to <125µm) without pigmentary changes.13 Intermediate AMD is in the presence of pigmentary abnormalities and medium or large size drusen (>125µm). The presence of GA or choroidal neovascularization is considered as late or advanced AMD (Figures 4 and 5).11 A simplified severity scale for AMD estimates the risk of conversion from dry to wet AMD to be 0.5% in the absence of large drusen and pigmentary changes.16 The risk of conversion from dry to wet AMD increases to 50% when both eyes demonstrate the presence of large drusen and pigmentary abnormalities.16
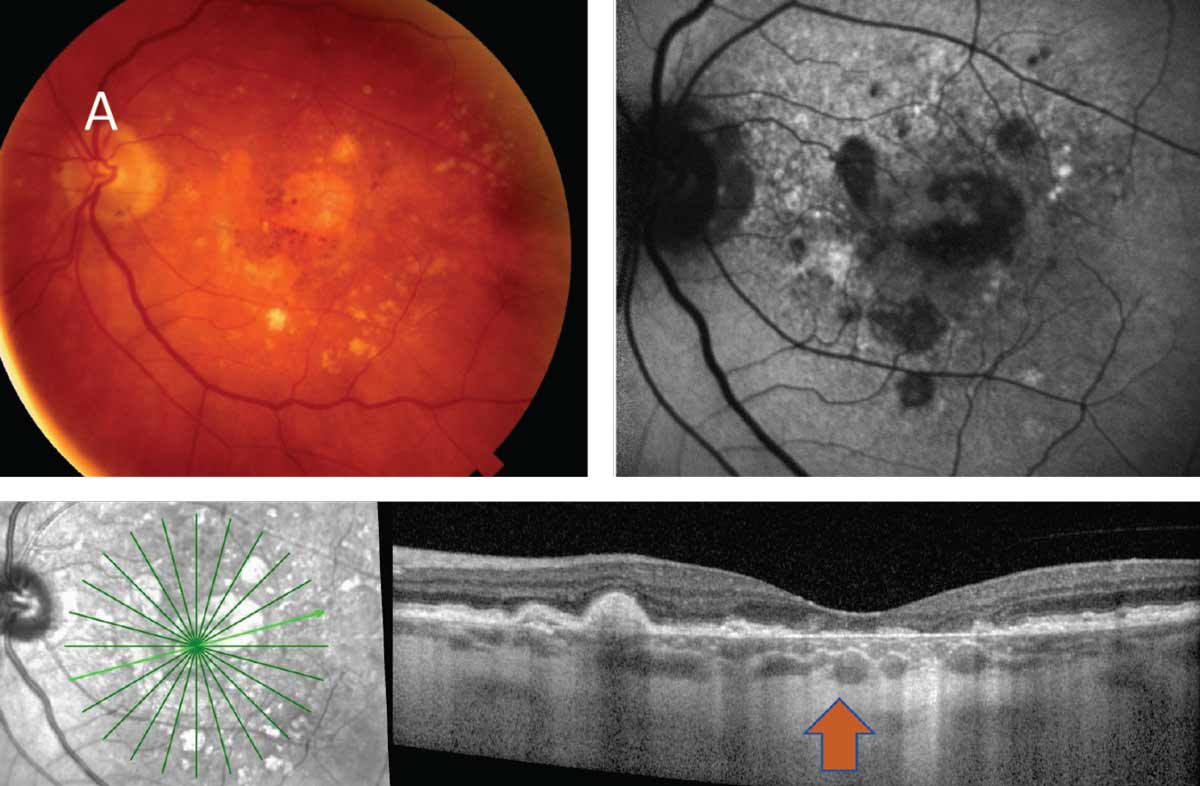 |
| Fig. 5. The clinical images above and below depict the two types of late or advanced macular degeneration. (A) Late or advanced atrophic AMD in the presence of GA. (B) Late or advanced wet AMD. Click image to enlarge. |
 |
| Click image to enlarge. |
Case 3
A 66-year-old Caucasian female complains of blurred vision. She has had cataract surgery five years earlier. She is a past smoker and has been on medication for systemic hypertension for several years. Her BCVA is 20/20 OD and 20/20 OS. At first glance, her fundus examination may appear to have several small drusen, as she had previously been given a diagnosis of early AMD. However, a closer look at her fundus photograph reveals subtle pigmentary abnormalities and presence of medium and large size drusen that can be confirmed on OCT. These findings allude to a diagnosis of intermediate AMD, the category with a higher risk for conversion to wet AMD (Figure 6).
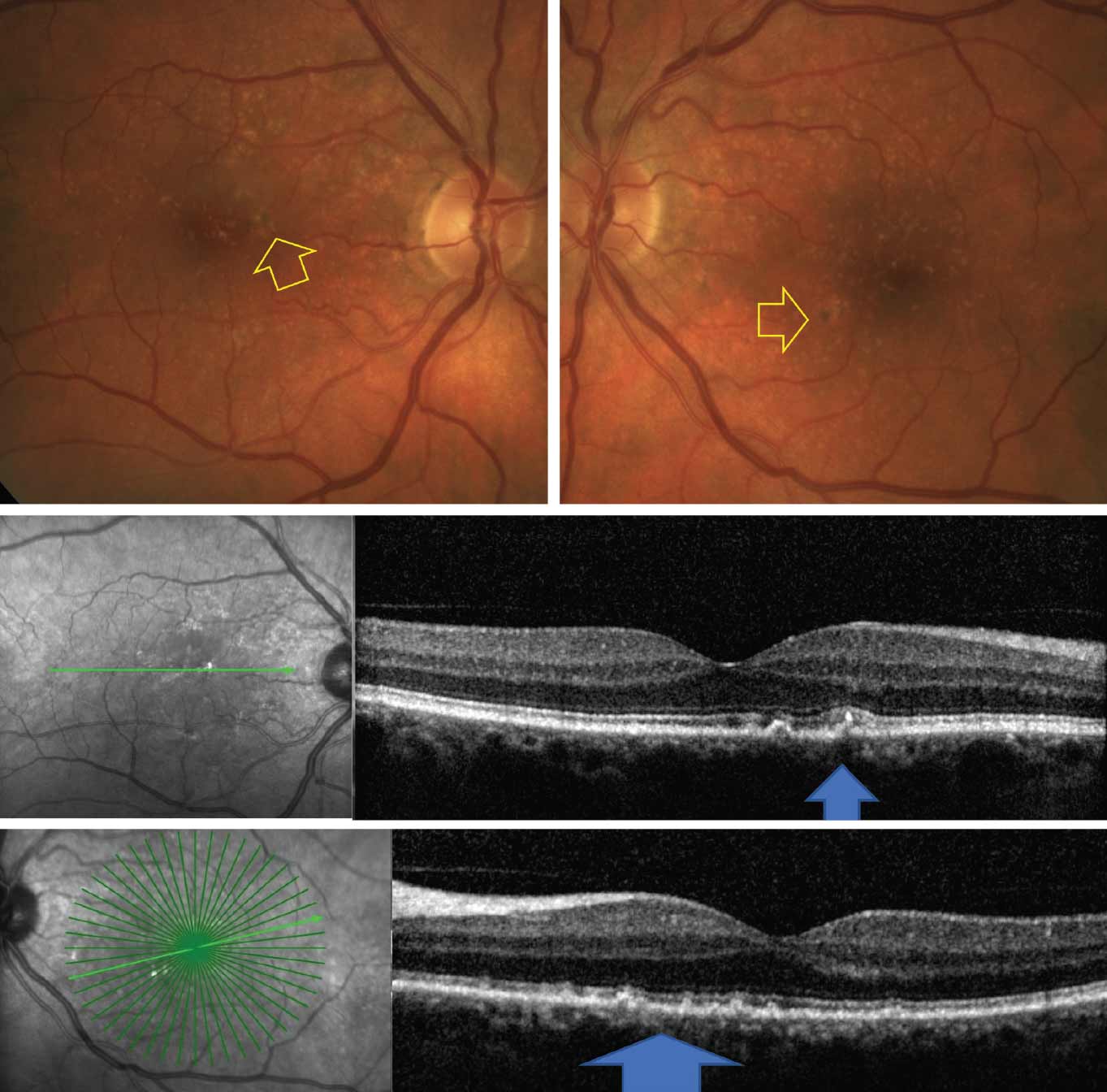 |
| Fig. 6. Fundus photos (top) show multiple drusen and pigmentary abnormalities (yellow arrow). OCT (bottom) shows medium and large size drusen (blue arrows). Click image to enlarge. |
Diagnostic Tools
AMD diagnosis involves a thorough ocular examination and includes a battery of specialized tests such as visual acuity assessment, dilated fundus examination and ancillary imaging modalities. Multimodal imaging plays a crucial role in timely diagnosis and appropriate staging of AMD. These include color fundus photography in either conventional white flash or the use of scanning laser ophthalmoscopy, FAF, multispectral imaging including infrared or near-infrared reflectance, OCT and OCT angiography. All of these imaging modalities are instrumental in the diagnosis of AMD.
In cases where AMD is suspected, supplementary diagnostic procedures such as fluorescein angiography or indocyanine green angiography may be employed to determine disease extent and guide appropriate management strategy (Figures 4 and 5).17 The earlier you detect the disease, make an accurate diagnosis based on clinical evidence and start the patient on treatment, the better their chances of avoiding the development of vision loss.
Management Options
In the early stage, patient education is the most important aspect of management. Communication regarding smoking cessation, improved dietary habits, monocular vision self-assessment, management of comorbidities, physical activity and exercise is essential. Additionally, patient understanding of potential consequences of disease progression and the need for regular annual eye examinations is a crucial component of patient education. In intermediate AMD, in addition to the above, vitamin and antioxidant supplementation as recommended by the AREDS and AREDS 2 studies should be discussed with the patient.18 Implementation of home monitoring devices such as Foreseehome (Notal Vision) has led to early detection at the time of conversion from dry to wet AMD, leading to better visual outcomes.19
For those patients who experience difficulties with daily living activities, low vision examination and rehabilitation are best considered earlier than later in the disease cycle.20
Management of late or advanced AMD is based on the neovascular or non-neovascular findings. The progression of early and intermediate AMD to GA or wet AMD varies based on different studies; however, its incidence is frequent enough to require patient surveillance for timely diagnosis and management.13,21,22 GA and wet AMD can also concurrently be present in the same eye and need to be independently treated.23
Medical Interventions
For the last two decades, the standard therapy for wet AMD has been intravitreal anti-VEGF injections. This began with the FDA approval of pegaptanib (Macugen) in 2004. Since then, a number of FDA-approved and one non-FDA-approved anti-VEGF have been used with good safety and efficacy for treatment of choroidal neovascularization caused by AMD and other conditions such as myopic degeneration.
Several other biosimilar agents either compatible with the current FDA-approved agents have either been approved or are in clinical trials.24 One downside of these agents is short durability, which results in requiring frequent injections for wet AMD, in some cases as often as monthly. Lapses in treatment have been shown to lead to poor outcomes.25 As a result, several strategies have been considered to reduce the injection burden that many patients experience. One of these is a recently FDA-approved biphasic anti-angiopoietin 2/anti-VEGF faricimab, Vabysmo (Genentech), that has increased durability and requires fewer injections.26
Another concept is the port delivery system (Susvimo; 100mg/mL ranibizumab injection, Genentech/Roche), which requires and implantation of a reservoir and can then be refilled every six months or longer. Following its 2021 FDA approval for wet AMD and during clinical trials for use of port delivery systems in the treatment of diabetic macular edema, the device had a voluntary recall by its developers with safety concerns occurring during refill procedures involving the device seal.27 Genentech/Roche have estimated that the product will return to the market by next year once the production issues are resolved.
Other alternatives to monthly injections in the pipeline include genetic modification by subretinal, suprachoroidal or intravitreal single injection of genetically modified viral vectors. A number of trials are ongoing to evaluate the viability of this modality as an alternative or augmentation to the monthly need for intravitreal injections.28
Another group of patients with advanced AMD are those suffering from GA, a progressive irreversible cause of central vision loss. On average, in 2.5 years, a small noncentral GA lesion can reach the foveal center resulting in significant loss of visual function, including decline in reading speed, ability to recognize faces and even inability to drive a car.29,30
Despite GA’s severity and imposed threat to visual function, until recently there were not any medical treatments for the condition. A number of novel and investigative agents had either failed to show efficacy or are involved in ongoing clinical trials. There is ample evidence of the role of genetic alteration in development of GA and choroidal neovascularization in AMD.31,32 Genetic variation in the complement genes have shown to be partly responsible in development of GA. Overreaction of the complement system, which is a part of human innate immune system, results in the development of the membrane attack complex, causing destruction of the RPE and photoreceptors.33
Based on the evidence, the inhibition of complements C5 and C3 have proven in clinical trials to result in slowing the progression of GA. Based on the results of clinical trials DERBY and OAKS, pegcetacoplan (Syfovre, Apellis Pharmaceutical), a C3 inhibitor, was recently FDA approved as an intravitreal injection for GA to be administered once every 25 to 60 days.34 Iveric Bio’s avacincaptad pegol, a C5 inhibitor, has also been shown to reduce growth of GA lesions in clinical trials GATHER 1 and 2 and is currently under review for FDA approval.35
With one therapy for GA on the market and others working their way through the pipeline, eyecare providers should be especially vigilant in identifying the condition to ensure appropriate management or referral of these patients.
Takeaways
AMD is a common condition seen by optometrists in the United States and worldwide, and its patient population is only growing larger with time. A significant number of patients can develop functional vision loss caused by various consequences of AMD. Various conditions can also mimic AMD, making it more difficult to properly diagnose and manage these patients.
To improve clinical and visual outcomes, optometrists should be well-versed in detecting and staging AMD, as well as know when to intervene and refer patients to retina subspecialty facilities for further assessment for treatment.
Dr. Rafieetary is a consultative optometrist at the Charles Retina Institute in Germantown, TN. He is a fellow of the American Academy of Optometry and the Optometric Retina Society. Dr. Rafieetary is on advisory boards for Heidelberg Engineering, Apellis, Iveric Bio, Notal Vision and OcuTerra. He is also a paid speaker for Optos, Heidelberg Engineering and Notal Vision.
Dr. Attar is an assistant professor in the Department of Ophthalmology at the University of Mississippi Medical Center. She is on advisory boards for Heidelberg Engineering, Apellis and OcuTerra Therapeutics.
1. Centers for Disease Control and Prevention. Prevalence of age-related macular degeneration (AMD). Updated October 31, 2022. www.cdc.gov/visionhealth/vehss/estimates/amd-prevalence.html. Accessed May 10, 2023. 2. Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122(4):598-614. 3. Janik-Papis K, Zaraś M, Skłodowska A, et al. Genetic aspects of age-related macular degeneration]. Klin Oczna. 2009;111(4-6):178-82. 4. Clemons TE, Milton RC, Klein R, Seddon JM, Ferris FL 3rd; AREDS Research Group. Risk factors for the incidence of advanced age-related macular degeneration in the Age-Related Eye Disease Study (AREDS) AREDS Report No. 19. Ophthalmology. 2005;112(4):533-9. 5. Friedman DS, Katz J, Bressler NM, Rahmani B, Tielsch JM. Racial differences in the prevalence of age-related macular degeneration: the Baltimore Eye Survey. Ophthalmology. 1999;106(6):1049-55. 6. Armstrong RA, Mousavi M. Overview of risk factors for age-related macular degeneration (AMD). J Stem Cells. 2015;10(3):171-91. 7. Neely DC, Bray KJ, Huisingh CE, et al. Prevalence of undiagnosed age-related macular degeneration in primary eye care. JAMA Ophthalmol. 2017;135(6):570-75. 8. Gelman R, Tsang SH. Masqueraders of age-related macular denegation, a number of inherited retinal diseases phenocopy AMD. Retina Today. 2011;65-70. 9. Kay DB, Land ME, Cooper RF, et al. Outer retinal structure in best vitelliform macular dystrophy. JAMA Ophthalmol. 2013;131(9):1207-15. 10. Tiosano L, Jaouni T, Averbukh E, Grunin M, Banin E, Chowers I. Bevacizumab treatment for choroidal neovascularization associated with adult-onset foveomacular vitelliform dystrophy. Eur J Ophthalmol. 2014;24(6):890-6. 11. Lindner M, Lambertus S, Mauschitz MM, et al. Differential disease progression in atrophic age-related macular degeneration and late-onset Stargardt disease. Invest Ophthalmol Vis Sci. 2017;58(2):1001-7. 12. Kassa E, Ciulla TA, Hussain RM, Dugel PU. Complement inhibition as a therapeutic strategy in retinal disorders. Expert Opin Biol Ther. 2019;19(4):335-42. 13. Ferris FL 3rd, Wilkinson CP, Bird A, et al; Beckman Initiative for Macular Research Classification Committee. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120(4):844-51. 14. Nigalye AK, Hess K, Pundlik SJ, et al. Dark adaptation and its role in age-related macular degeneration. J Clin Med. 2022;11(5):1358. 15. Sigler EJ, Randolph JC. Comparison of macular choroidal thickness among patients older than age 65 with early atrophic age-related macular degeneration and normals. Invest Ophthalmol Vis Sci. 2013;54(9):6307-13. 16. Ferris FL, Davis MD, Clemons TE, et al; AREDS Research Group. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch Ophthalmol. 2005;123(11):1570-4. 17. Jaffe GJ, Chakravarthy U, Freund KB, et al. Imaging features associated with progression to geographic atrophy in age-related macular degeneration: Classification of Atrophy Meeting Report 5. Ophthalmol Retina. 2021;5(9):855-67. 18. Chew EY, Clemons T, SanGiovanni JP, et al; AREDS2 Research Group. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 Report No. 1). Ophthalmology. 2012;119(11):2282-9. 19. Mathai M, Reddy S, Elman MJ, et al; ALOFT study group. Analysis of the long-term visual outcomes of ForeseeHome remote telemonitoring: the ALOFT Study. Ophthalmol Retina. 2022;6(10):922-9. 20. Gopalakrishnan S, Velu S, Raman R. Low-vision intervention in individuals with age-related macular degeneration. Indian J Ophthalmol. 2020;68(5):886-9. 21. Chakravarthy U, Bailey CC, Scanlon PH, et al. Progression from early/intermediate to advanced forms of age-related macular degeneration in a large UK cohort: rates and risk factors. Ophthalmol Retina. 2020;4(7):662-72. 22. Xu L, Mrejen S, Jung JJ, et al. Geographic atrophy in patients receiving anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Retina. 2015;35(2):176-86. 23. Kaszubski P, Ben Ami T, Saade C, Smith RT. Geographic atrophy and choroidal neovascularization in the same eye: a review. Ophthalmic Res. 2016;55(4):185-93. 24. Kapur M, Nirula S, Naik MP. Future of anti-VEGF: biosimilars and biobetters. Int J Retina Vitreous. 2022;8(1):2. 25. Greenlee TE, Wang VY, Kang H, et al. Consequences of lapses in treatment with vascular endothelial growth factor inhibitors in neovascular age-related macular degeneration in routine clinical practice. Retina. 2021;41(3):581-7. 26. Nicolò M, Ferro Desideri L, Vagge A, Traverso CE. Faricimab: an investigational agent targeting the Tie-2/angiopoietin pathway and VEGF-A for the treatment of retinal diseases. Expert Opin Investig Drugs. 2021;30(3):193-200. 27. Sharma A, Khanani AM, Parachuri N, et al. Port delivery system with ranibizumab (Susvimo) recall- what does it mean to the retina specialists. Int J Retina Vitreous. 2023;9(1):6. 28. Rafieetary S, Huddleston S. Gene therapy delivery: Examining the evidence. Retina Specialist. March/April 2023;24-27. 29. Lindblad AS, Lloyd PC, Clemons TE, et al; AREDS Research Group. Change in area of geographic atrophy in the Age-Related Eye Disease Study: AREDS Report No. 26. Arch Ophthalmol. 2009;127(9):1168-74. 30. Patel PJ, Ziemssen F, Ng E, et al. Burden of illness in geographic atrophy: a study of vision-related quality of life and health care resource use. Clin Ophthalmol. 2020;14:15-28. 31. Yan Q, Ding Y, Liu Y, et al. Genome-wide analysis of disease progression in age-related macular degeneration. Hum Mol Genet. 2018;27(5):929-40. 32. Fritsche LG, Igl W, Bailey JN, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016;48(2):134-43. 33. Katschke KJ Jr, Xi H, Cox C, et al. Classical and alternative complement activation on photoreceptor outer segments drives monocyte-dependent retinal atrophy. Sci Rep. 2018;8(1):7348. 34. Goldberg R, Heier JS, Wykoff CC, et al. Efficacy of intravitreal pegcetacoplan in patients with geographic atrophy (GA): 12-month results from the phase 3 OAKS and DERBY studies. Invest Ophthalmol Vis Sci. 2022;63(7):1500. 35. Jaffe GJ, Westby K, Csaky KG, et al. C5 Inhibitor avacincaptad pegol for geographic atrophy due to age-related macular degeneration: a randomized pivotal phase 2/3 trial. Ophthalmology. 2021;128(4):576-86. |