As the final remnants of winter thaw and the new season takes hold, we anticipate the growth and renewal that comes with this time of year. Spring brings a slow but steady increase in outdoor activity, and the inevitable arrival of the first blooms of flowers and trees—followed quickly by the first sneezes and eye rubs of yet another allergy season.
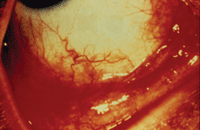
Patients with chronic allergic conjunctivitis may experience itching, redness and burning of the eyes as well as excessive tearing.
Seasonal and perennial allergies are a significant global health issue affecting approximately 15% of the world’s population; these percentages are even higher in the industrialized countries of Western Europe, Eastern Asia, Australia and North America.1 In the United States, seasonal and perennial allergies affect 20% of the population, and 70% to 80% of these patients report that their allergies include ocular symptoms.1,2 While not life threatening, the symptoms of ocular allergy can have a significant impact on quality of life for those who experience them.
Ocular allergy, or allergic conjunctivitis (AC), is typically cited as one of the most frequent reasons patients seek medical treatment for seasonal allergies.2,3 Recent estimates put the annual economic impact of seasonal allergies in the United States at $7 billion, including almost $1 billion in work absenteeism.2
While there have been improvements in therapy over the past two decades, there has also been an increase in prevalence of the disease.2,3 In addition, most of the drugs currently available to treat AC target ocular itching, leaving other signs and symptoms, such as ocular redness and chronic inflammation, untreated or under-treated.
As we prepare for this coming season, let’s take a look at the current treatments for ocular allergies, review the therapeutic developments in years past and describe areas where future efforts could have the most impact.
Changes in Treatment
Specific pharmacological treatments for ocular allergy were non-existent prior to the early 1970s; typically, patients were instructed to use cold compresses and to avoid the offending allergen.4,6 The first topical agents developed to treat AC were adrenergic agonists (such as naphazoline)—these drugs were effective at reducing hyperemia but did little to treat ocular itching.9
A second group of drugs, the mast cell stabilizers, first became available for topical ophthalmic use in the 1980s.10,11 While these drugs showed efficacy in reducing ocular itch, they were relatively short-acting, and were also limited by the nature of their mechanism of action; because they are preventive treatments, they must be taken before the allergen is present to be effective.12
As we discuss below, patients typically used anti-allergics as needed, regardless of label (or practitioner) instructions and so, for the mast cell stabilizers, the arrival of symptoms is too late for drug treatment.
The first topical H1-antihistamines for ocular use (pheniramine and antazoline) came to market in 1990.13,14 These were relatively short-acting medications, but were marketed as topical combinations of antihistamines and adrenergic agonists, creating a formulation that could treat both ocular itch and ocular redness. Some of these formulations are still available today as non-prescription topicals, but they have been replaced in recent years by superior, single-agent antihistamines.
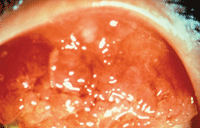
The patient shown has severe vernal conjunctivitis, with a marked papillary response.
In the last two decades, a number of second-generation H1-antihistamines have been developed to improve upon earlier drugs in terms of duration of action, safety profile and comfort. Important drugs in this group include levocabastine hydrochloride and emedastine difumarate, both of which were available as single-agent topicals.15-17 While still requiring multiple daily dosing, both of these agents are highly effective in reducing ocular itch.
A progression of antihistamines followed, including azelastine hydrochloride, bepotastine besilate, ketotifen fumarate and olopatadine hydrochloride.18-21 Each of these compounds is classified as a “dual-action” antihistamine because they exhibit both H1 receptor antagonism and mast cell stabilization effects.12
In addition to this dual-action effect (or perhaps because of it), these newer drugs all have a longer duration of action than either levocabastine or emedastine. They are indicated for twice-daily dosing to relieve itching due to AC.
Most recently, two agents have been approved for once-daily dosing—a higher concentration formula (0.2%) of olopatadine hydrochloride, (Pataday, Alcon), and the newest ocular antihistamine, alcaftadine (Lastacaft, Allergan).22,23
Like other allergies, AC is caused by a type-1 (IgE-dependent) hypersensitivity reaction.2 Exposure to allergens triggers mast cell release of inflammatory mediators, including histamine, that induce symptoms such as ocular itching and hyperemia.4,5 Additional symptoms include swelling of the surrounding eyelids, chemosis and tearing.4-6 Conjunctival swab cultures provide an unequivocal differential diagnosis of an infectious vs. allergic etiology, but the key feature that distinguishes AC from other forms of conjunctivitis is itching.
More severe, chronic forms of ocular allergy include rare conditions such as atopic keratoconjunctivitis (AKC), vernal keratoconjunctivitis (VKC) and giant papillary conjunctivitis (GPC).6-8 Both AKC and VKC are allergic conditions in which the inflammatory response has been exacerbated to the point of significant conjunctival erythema and significant risk of corneal ulceration. In contrast, GPC is a condition specifically associated with contact lens use, and is due to a response to allergens deposited in the contact. All three of these conditions tend to be chronic, and are typically treated with a combination of antihistamines, mast cell stabilizers and topical steroids.
A noteworthy aspect of histamine antagonists is that, unlike the pure mast cell stabilizers, these drugs are effective whenever the patients experience allergic symptoms. This was a significant step forward, and many would say it changed the landscape of ocular allergy therapy. In addition, the high efficacy of these drugs meant that most patients moved from an everyday, “prevention-based” dosing to a more “as-needed” use.
Causes and Conditions
Ironically, it’s possible that antihistamine/mast cell stabilizers could be more effective if used prophylactically; evidence suggests that prevention of acute allergic responses may be one way to minimize the growing trend toward chronic allergies.24 Daily use of topical antihistamine/mast cell stabilizers during allergy season would be likely to have such an effect.
Treating Poor Responders
Despite this continued improvement in AC therapy, many patients with ocular allergies (some estimates put the number at 30% in the U.S.) show poor response to most currently available therapies.1 These poor responders to antihistamine therapy appear to fall into two groups: chronic allergics and seasonal allergics.
The first group consists of those with the combination of seasonal and perennial allergies; for these patients, it is always allergy season. The second group includes patients with robust responses to seasonal allergens, so that on days with particularly high pollen levels they present an allergic response that simply overwhelms the ability of any topical antihistamine to suppress.
Both types involve conjunctival recruitment of immune cells in addition to mast cells, and so the goal of any new therapy is to “calm” the conjunctiva, allow the recruited cells time to dissipate and also reduce the inflammatory features of the chronic, late phase response. These patients can be considered chronic ocular allergy sufferers, and they are symptomatically similar to those with more severe allergic conditions such as AKC.
Chronic allergy differs from the more acute forms in that it is primarily mediated by cellular factors, and is dependent upon the activity of immune cells such as basophils and eosinophils that have infiltrated the conjunctiva over the course of prolonged allergen exposure.24 The increased prevalence of chronic atopic diseases such as AC in recent years, especially in more industrialized countries, is thought to be a result of increased exposure to allergy-exacerbating agents, such as air pollutants and volatile chemicals.
Evidence suggests that these chemicals can prime the immune response to perennial allergens, such as dust mites and molds.25 As the prevalence of these chronic poor responders increases, current and future anti-allergic drug development must identify therapies to address this unmet need.
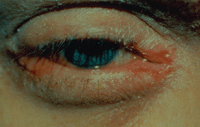
With atopic keratoconjunctivitis, the lower eyelid is typically affected more than the upper lid and the conjunctiva lining the eyelids is usually red and swollen.
Currently, the best available treatments for chronic ocular allergy sufferers are topical steroids, such as prednisolone acetate or loteprednol etabonate.12 While effective, these drugs are typically used for brief periods (courses of one to two weeks) to minimize the risk of adverse ocular effects such as cataracts or increases in intraocular pressure.
Newer anti-inflammatory compounds are likely candidates for future studies of chronic AC. Beyond trials of compounds with theoretical or demonstrable anti-inflammatory effects, however, it is necessary to establish a clear strategy for identifying and developing the next class of ocular anti-allergics.
Looking Ahead
Researchers at Ora, Inc. have spent the past 30 years developing and refining methods to test new drugs and formulations for ocular allergy. In that time, our conjunctival allergen challenge (CAC) model has become an industry standard, and has been employed for studies used to gain FDA approval for all ocular anti-allergics currently marketed in the U.S.26 Recently, Japan’s Pharmaceuticals and Medical Devices Agency also adopted the CAC model for allergic drug development. This means that testing of new anti-allergics for both the American and Japanese markets can be conducted simultaneously, and should speed drug development.
The success of clinical models such as the CAC underscores the fundamental importance of study design in the drug development process. The research group at Ora, like others in the ocular therapeutics industry, is focused on how to accurately assess the efficacy of either new chemical entities or repurposed drugs as therapies for chronic ocular allergy. Key to these efforts is the ability to accurately identify the “non-responder” population from the greater population of allergics.
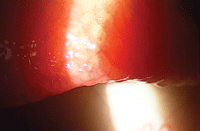
Patients with asthma, hay fever or animal allergies may be at greater risk for GPC; its etiology may be immunological, where contact lens deposits act as allergens. Photo: Paul M. Karpecki, O.D.
In addition, trials need to employ robust experimental standards that elicit the chronic allergic signs and symptoms, similar to the CAC model for acute allergy. Future therapies will likely employ drugs that interfere with cytokine signaling, or those that can disrupt the intracellular processes that mediate this chronic feedback loop.
Results of pre-clinical studies have focused on a number of factors that define the chronic allergic subject. Prolonged or high levels of allergen exposure lead to infiltration and accumulation of basophils, eosinophils and increased numbers of mast cells. These cells respond to continued presence of allergens by releasing a smorgasbord of cytokines, chemo-attractants, proteases and other signaling molecules. The net effect is continued recruitment of immune and inflammatory cells, breakdown of the ocular surface’s extracellular matrix and destabilization of the protective, barrier function of the conjunctival and corneal epithelium.24
Mr. Gomes is vice president of Allergy at Ora, Inc. Ora has provided clinical research services for each product mentioned.
1. Singh K, Axelrod S, Bielory L. The epidemiology of ocular and nasal allergy in the United States, 1988-1994. J Allergy Clin Immunol. 2010 Oct;126(4):778-83.e6.
2. Blaiss MS. Allergic rhinoconjunctivitis: burden of disease. Allergy Asthma Proc. 2007 Jul-Aug; 28(4):393-7.
3. Ciprandi G, Buscaglia S, Cerqueti PM, Canonica GW. Drug treatment of allergic conjunctivitis. A review of the evidence. Drugs. 1992 Feb; 43(2):154-76.
4. Collum LT, Kilmartin DJ. Acute allergic conjunctivitis. In: Allergic Diseases of the Eye. Philadelphia: W.B. Saunders Co.;2000:108-32.
5. Akdis CA, Blaser K. Histamine in the immune regulation of allergic inflammation. J Allergy Clin Immunol. 2003 Jul;112(1):15-22.
6. Abelson MB, Smith L, Chapin M. Ocular allergic disease: mechanisms, disease sub-types, treatment. Ocul Surf. 2003 Jul; 1(3):127-49.
7. Butrus S, Portela R. Ocular allergy: diagnosis and treatment. Ophthalmol Clin North Am. 2005 Dec;18(4):485-92.
8. Leonardi A. Emerging drugs for ocular allergy. Expert Opin Emerg Drugs. 2005 Aug;10(3):505-20.
9. Abelson MB, Yamamoto GK, Allansmith MR. Effects of ocular decongestants. Arch Ophthalmol. 1980 May; 98(5):856-8.
10. Abelson MB, Wun PJ, Nevius JM. Mast cell stabilizers. In: Allergic Diseases of the Eye. Philadelphia: W.B. Saunders Co.;2000:228-34.
11. Cook EB, Stahl JL, Barney NP, Graziano FM. Mechanisms of antihistamines and mast cell stabilizers in ocular allergic inflammation. Curr Drug Targets Inflamm Allergy. 2002 Jun;1(2):167-80.
12. Abramowicz M, ed. Drugs for allergic disorders. ” Medical Letter Treatment Guidelines. 2010;8(90):9-18.
13. Dockhorn RJ, Duckett TG. Comparison of Naphcon-A and its components (naphazoline and pheniramine) in a provocative model of allergic conjunctivitis. Curr Eye Res. 1994 May;13(5):319-24.
14. Abelson MB, Paradis A, George MA, et al. Effects of Vasocon-A in the allergen challenge model of acute allergic conjunctivitis. Arch Ophthalmol. 1990 Apr;108(4):520-4.
15. Abelson MB, George MA, Schaefer K, Smith LM. Evaluation of the new ophthalmic antihistamine, 0.05% levocabastine, in the clinical allergen challenge model of allergic conjunctivitis. J Allergy Clin Immunol. 1994 Sep;94(3 Pt 1):458-64.
16. Abelson MB, Kaplan AP. A randomized, double-blind, placebo-controlled comparison of emedastine 0.05% ophthalmic solution with loratadine 10 mg and their combination in the human conjunctival allergen challenge model. Clin Ther. 2002 Mar; 24(3):445-56.
17. Secchi A, Ciprandi G, Leonardi A, et al. Safety and efficacy comparison of emedastine 0.05% ophthalmic solution compared to levocabastine 0.05% ophthalmic suspension in pediatric subjects with allergic conjunctivitis. Emadine Study Group. Acta Ophthalmol Scand Suppl. 2000;230:42-7.
18. Giede C, Metzenauer P, Petzold U, Ellers-Lenz B. Comparison of azelastine eye drops with levocabastine eye drops in the treatment of seasonal allergic conjunctivitis. Curr Med Res Opin. 2000;16(3):153-63.
19. Greiner JV, Mundorf T, Dubiner H, et al. Efficacy and safety of ketotifen fumarate 0.025% in the conjunctival antigen challenge model of ocular allergic conjunctivitis. Am J Ophthalmol. 2003 Dec;136(6):1097-105.
20. Sharif NA, Xu SX, Miller ST, et al. Characterization of the ocular antiallergic and antihistaminic effects of olopatadine (AL-4943A), a novel drug for treating ocular allergic diseases. J Pharmacol Exp Ther. 1996 Sep;278(3):1252-61.
21. Macejko TT, Bergmann MT, Williams JI, et al. Multicenter clinical evaluation of bepotastine besilate ophthalmic solutions 1.0% and 1.5% to treat allergic conjunctivitis. Am J Ophthalmol. 2010 Jul;150(1):122-27.e5.
22. Abelson MB, Spangler DL, Epstein AB, et al. Efficacy of once-daily olopatadine 0.2% ophthalmic solution compared to twice-daily olopatadine 0.1% ophthalmic solution for the treatment of ocular itching induced by conjunctival allergen challenge. Curr Eye Res. 2007 Dec; 32(12):1017-22.
23. Torkildsen G, Shedden A. The safety and efficacy of alcaftadine 0.25% ophthalmic solution for the prevention of itching associated with allergic conjunctivitis. Curr Med Res Opin. 2011 Mar;27(3):623-31.
24. Choi SH, Beilory L. Late-phase reaction in ocular allergy. Curr Opin Allergy Clin Immunol. 2008 Oct;8(5):438-44.
25. Riediker M, Monn C, Koller T, et al. Air pollutants enhance rhinoconjunctivitis symptoms in pollen-allergic individuals. Ann Allergy Asthma Immunol. 2001 Oct;87(4):311-8.
26. Abelson MB, Chambers WA, Smith LM. Conjunctival allergen challenge. A clinical approach to studying allergic conjunctivitis. Arch Ophthalmol. 1990 Jan;108(1):84-8.

