Although several types of sleep-disordered breathing exist, obstructive sleep apnea (OSA) is certainly the most common and the most publicized.1 The condition is caused by a complete or partial anatomical upper airway collapse that temporally restricts or obstructs breathing, often in a cyclical pattern. The reduction of breathing is hypopnea, the cessation of breathing is apnea. This respiratory disruption reduces blood oxyhemoglobin saturation and impacts blood pressure, heart rate, sympathetic activity, metabolic activity and sleep. It elevates an individual’s risk for hypertension, coronary artery disease, myocardial infarction, congestive heart failure and stroke.
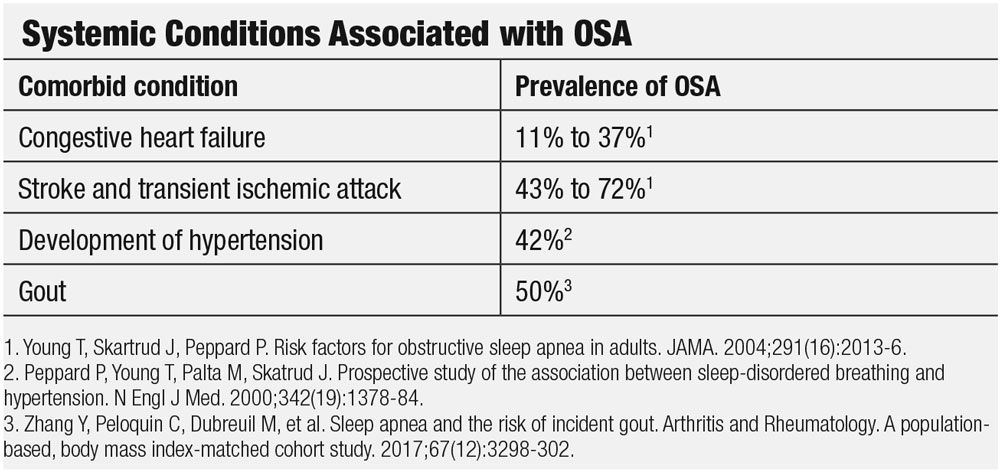
OSA patients may experience cognitive dysfunction, depression and the sleep disturbances may even trigger metabolic syndrome and lead to diabetes.2,3 Additionally, the risk for gout is increased by two times in older patients and OSA is a risk factor for dislipidemas.3,4 As you can see, OSA and the disordered sleep it causes can have a deleterious impact on literally every part of the human body, and the eyes are no exception. In fact, OSA is associated with several ocular conditions, from the anterior to posterior segment and impacting a wide range of structures from the tear film to the optic nerve.
Here, we review the risk factors for OSA, how to recognize and diagnose an OSA patient and how the optometrist can comanage these patients, with special attention to its associated ocular diseases.
 |
| Vogt’s striae, as seen here, can indicate keratoconus, which is more common in patients with OSA than those without. |
Diagnosis
The main risk factor for OSA is obesity (which can double the risk), specifically a neck circumference greater than 17 inches.2,5,6 It is more prevalent in males, with females catching up following menopause. Aging, craniofacial deformities, smoking and alcohol use before bed are also associated with OSA.2
Most patients with OSA have no problems breathing while awake, but during sleep, muscle tone relaxes and soft tissue in the pharynx collapses to obstruct the airway. It’s the obesity that increases the soft tissue (adipose) around the pharynx combined with a decrease in muscle tone with age—OSA is two to three times more prevalent in patients older than 65.2 While a complex neurochemical feedback mechanism exists to promote breathing when blood CO2 rises, the stimuli is insufficient to override the soft tissue obstruction when the airway is compromised. The impaired feedback mechanism will result in imprecise ventilation undershoots and overshoots with sudden neurological arousals to promote breathing which disrupt the sleep cycle.7 While questionnaires can be helpful for screening, OSA is formally diagnosed with polysomnography, or a continuous overnight sleep study, where the patient’s breathing, heart rate, brain waves, blood oxygen levels and other parameters are monitored.2,6
Sleeping with OSA is characterized by loud snoring, periods of not breathing, followed by gasps when breathing resumes.2 People who sleep alone may be unaware of their breathing disorder, and may become habituated to the daytime sleepiness and fatigue that results from fragmented sleep. People in relationships are more likely to be diagnosed with OSA as they are more likely to be informed of their sleep breathing disorder (SBD).2 Even so, approximately 78% to 80% of the OSA population who could benefit from treatment go undiagnosed.2 Furthermore, as the American population becomes more obese, the rate of sleep disordered breathing in the American population continues to rise. One study using data from 2007 to 2010 estimates that the rate of SBD in American adults aged 30 to 70 is 14% in men and 5% in women with symptoms that meet the Medicare criteria for OSA, which is a significant increase compared with data collected from 1988 to 1994.6
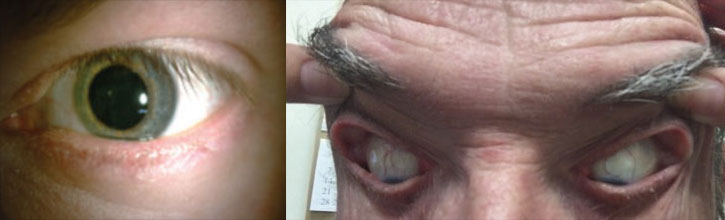 |
| Patients with OSA may experience a variety of lid and lash conditions such as lash ptosis, at left, and floppy eyelid syndrome, which is characterized by superior lids that are easily everted with minimal lateral force, as shown right. Photos by Victoria Roan, OD. |
Ocular Associations
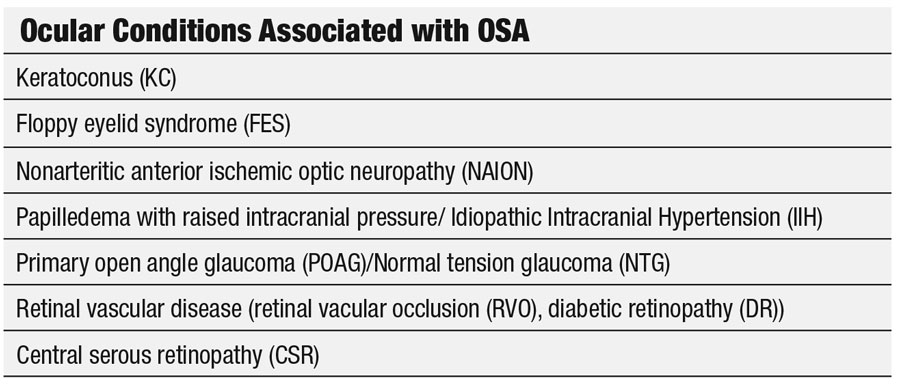
OSA is associated with several eye conditions. In 2005, investigators reported an association with floppy eyelid syndrome (FES), primary open angle glaucoma (POAG), normal-tension glaucoma (NTG), optic neuropathy, nonarteritic anterior ischemic optic neuropathy (NAION) and papilledema with raised intracranial pressure (ICP).8 Additional research shows an association with keratoconus and an increase in diabetic retinopathy (DR), especially proliferative diabetic retinopathy (PDR) as well as central serous retinopathy.9-11 Research shows OSA’s impact on an array of structures within the eye, including the lids, the cornea, the optic nerves, and the retina.
Anterior segment and lids. In a population of patients with keratoconus, 18% had previously been diagnosed with OSA with sleep testing and an additional 47% of patients at high risk for developing OSA, according to a 2012 study.9 Of note, the average BMI of the patients in this study was 31.2.9
Another study focused on FES, a condition characterized by an elastic-like upper eyelid that is easily pliant and everted during sleep or manually with minimal lateral traction. The study found 16 out of 17 patients with OSA had FES.8 This high correlation suggests that every patient with OSA should have their lids everted to check for FES related papillary conjunctivitis.
Any patient diagnosed with FES who has not been diagnosed with OSA should be referred to their primary care physician for a work-up, or a cardiologist, neurologist, or pulmonologist if the patient is currently under their care. The rubbery tarsal plate in FES fails to support the eyelid platform properly, with the eyelashes pointing downwards over the visual axis, making the presence of lash ptosis a red flag.12 Differential diagnosis includes other types of chronic papillary conjunctivitis. FES patients tend to be obese males with OSA.
For any acute corneal or conjunctival insult, eye care providers may apply an ophthalmic ointment, such as erythromycin, and switch to a lubricating ointment when the lesions resolve. Protecting the eyes during sleep by taping the eyelids or using a tightly-fitting eye mask or shields may be indicated. For severe cases, surgical tightening of the eyelids is an option.13
Optic nerves and glaucoma. Researchers believe OSA can damage the vascular and mechanical structures within the second cranial nerve. Among other factors, the recurrent impaired nocturnal vascular perfusion caused by OSA may damage the optic nerve, while raised nocturnal intracranial pressure and elastic fiber depletion can cause mechanical damage.14
NTG patients frequently have OSA issues, according to one study showing that a large percentage of middle aged or older NTG patients test positive for OSA with polysomnography. Researchers hypothesize that impaired autoregulation of optic disc circulation results in nerve damage.15 NTG is more highly associated with and OSA than with POAG.
 |
NAION patients with OSA experience nocturnal hypoxia, which may result in episodic hypotension. Also, the nocturnal hypoxia may directly damage the optic nerve. Investigators point to an exceptionally high association with NAION and OSA, with 70% of NAION patients having OSA. When referring NAOIN patients, be sure to mention the association with OSA.16
Papilledemas can include pseudopapilledema, papillitis, ischemic optic neuropathy and hypertensive optic neuropathy among others. All involve optic disc swelling caused by raised intracranial pressure (ICP). Since papilledema can sometimes be caused by an intracranial mass, emergency cranial imaging is indicated.17 The nocturnal hypoxia caused by OSA changes the cerebral vasculature which itself is associated with elevated ICP.18
Idiopathic intracranial hypertension (IIH), also called pseudotumor cerebri, is a condition that primarily impacts females with obesity. It is also associated with OSA and disordered sleep.19 Signs include increased ICP, normal brain imaging and increased opening pressure on lumbar puncture with normal cerebral spinal fluid findings and an enlarged blind spot. Symptoms for patients with IIH and elevated ICP are similar to the symptoms of those with papilledema with the addition of pulsatile tinnitus. Acetazolamide is often prescribed if there is no sulfa allergy, whether the patient is symptomatic or not.20 The Journal of Clinical Sleep Medicine reports on one patient treated with both acetazolamide and CPAP who showed resolution of papilledema.21 Another study suggested that using the Berlin questionnaire would be a practical tool to direct IIH patients at high risk for OSA for polysomnography.22
Retina and vasculature. Retinal vascular occlusion (RVO) includes sudden, painless vision loss that is usually unilateral, with visual field defects. Retinal hemorrhages and dilated, tortuous veins are often seen.23 Symptoms of RVO usually manifest upon waking similar to NAION, suggesting microvascular and hypercoagulable changes during nocturnal apnea events. Research shows that patients with RVO often have OSA, and OSA is now considered to be a risk factor for RVO events.1
Diabetic retinopathy (DR). The hyperglycemia caused by diabetes leads to oxidative stress and damage to the vascular endothelium, resulting in permeable blood vessels.24 Early nonproliferative diabetic retinopathy (NPDR) is characterized by retinal hemorrhages and microaneurisms. The leaky blood vessels allow the deposition of hard exudates (lipids) and fluid in the macula resulting in diabetic macular edema (DME) and vision loss. Progressive capillary nonperfusion resulting in ischemia will promote the formation of PDR which is characterized by the proliferation of neovascularization on the surface of the retina or optic disc. PDR can result in vitreous hemorrhages, fibrosis and tractional detachments.25 In a study of diabetic patients who already had DR, PDR was much more prevalent in patients with OSA.10 Vascular endothelial growth factor (VEGF) is sensitive to hypoxia and responsible for new blood vessel production.26 Nocturnal desaturation and reoxygenation caused by OSA was thought to be directly related to the increased development of PDR in these patients.10 The management of diabetic retinopathy includes tight glycemic control, panretinal and focal laser photocoagulation, anti-VEGF therapy and vitrectomy.25,27
Central serous retinopathy (CSR) is an idiopathic serous retinopathy characterized by blurred vision, metamorphopisia and a central blind spot. The patient presents with a serous detachment of the neurosensory retina in the macula. The classic patient profile is a male between the ages of 25 and 50 who is stressed, which is why an association with cortisol is suspected.28 The constant interruption of the sleep cycle in OSA affects the sympathetic system and promotes an increased production of circulating norepinephrine and epinephrine which is thought to increase vascular permeability leading to serous fluid leakage.11
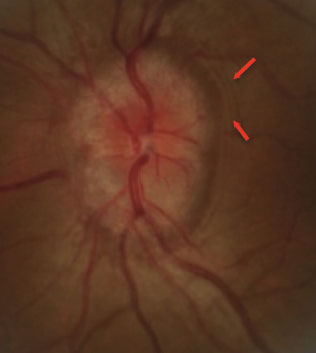 |
| This pediatric patient’s papilledema is evident due to the microvascular dilation of the optic nerve, vessel obscuration and Paton’s line (red arrows). Papilledema is associated with OSA. Photo by Diana Shechtman, OD. |
Systemic Treatment
Before 1981, the only treatment for OSA was a tracheostomy, a surgically created hole with a tube leading through the front of the neck and into trachea, created by a tracheotomy.2 This invasive procedure adversely affects the ability to speak and impacts eating. The patient is also burdened by the care of the “trach tube” to prevent clogging and infection.29
All of this changed when the continuous positive airway pressure (CPAP) ventilator was developed, which is now considered standard in OSA treatment.30 A machine provides constant airflow, and the patient wears a mask over the nose or mouth or both to direct the flow through the airway during sleep. This continuous pushing of air through the trachea keeps the airway open and prevents the airway collapse experienced in OSA. Research shows that CPAP usage reduces airway obstructions and improves sleep in patients with OSA, reducing daytime sleepiness and other systemic impacts.31
CPAP usage is not without its own issues, as some patients cannot tolerate wearing a mask during sleep or develop a dry nose or other issues. CPAP usage can also have deleterious effects on the eyes, also, which will be discussed later. Other OSA treatments include mouth guards or splints which widen the airway by pushing the jaw forward and advancing the tongue, but these are not as effective as CPAP. Reconstructive surgery to the upper airway to improve airflow has also been performed, but the effectiveness of such procedures is not well studied.31
CPAP usage needs to be long-term which is impacted by poor adherence by users. The benefits of extended usage cannot be ignored—CPAP usage can reduce snoring and nocturnal awakenings which improves sleeping.32 The associated reduction in daytime sleepiness has also been associated with a decrease in motor vehicle accidents attributed to OSA.32 CPAP usage seems to have its largest impact on cardiovascular outcomes and hypertension. Metabolic syndrome and hyperlipidemia is also improved.32 The sleep fragmentation in OSA seems to primarily affect attention/vigilance while hypoxia is linked to global cognitive function.33 A meta-review of CPAP treatment on cognitive function showed medium to large improvements in five subcategories of executive function.34
Due to the strong association with obesity, lifestyle changes are encouraged for those patients with OSA who are obese—defined as any body mass index (BMI) of greater than 30. While diet and exercise leading to weight loss can help reduce the severity of OSA, it is thought that weight reduction alone may be insufficient to reverse the condition.35 Cessation of smoking along with the reduction of nighttime alcohol use are also encouraged.31
 |
CPAP Impact
If the ocular impacts of OSA weren’t enough, CPAP usage also can have a deleterious effect on the eye. Air blown into the eyes from poor fitting CPAP masks has long been known to cause ocular dryness. Conjunctivitis resulting from CPAP use was reported as early as 1984.36 One paper identified anterior segment problems such as dryness and recurrent corneal infections observed in contact lens wearers being treated with CPAP.37
Improvements in CPAP mask fitting can reduce air leakage, and the importance of keeping the CPAP disinfected is better understood, but dryness can still result from air being pushed up through the nose into the nasal lacrimal canal, especially with those who have nasal lacrimal tubes.38,39 If improvement in dryness cannot be attained with changes in the mask fit, punctal plugs may be used to help with air reflux.40 In addition, besides the aforementioned association of OSA with glaucoma, CPAP use is also linked to elevated intracocular pressure, making the monitoring of OSA patients who are glaucomatous or glaucoma suspects who are treated with CPAP even more critical.41
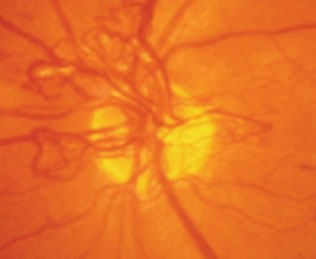 |
| Patients with concurrent diabetes and OSA who already have diabetic retinopathy are more likely to progress to proliferative diabetic retinopathy, as seen here, than those without OSA. Photo by Paul Chous, OD. |
OSA is a prevalent condition that is often underdiagnosed and that has serious health implications. Pay special attention to patients who have keratoconus, FES, lash ptosis, papilledema and vascular issues. Question those known to have OSA about their treatment protocol, especially if they’re using a CPAP. Patients are not always forthcoming about this and may not be aware of its potential impact on their eyes. Ask them about any dry eye issues and consider testing them for ocular dryness. Unusual or unexplained recurrent bacterial conjunctivitis could also be a possible indicator of CPAP complications.
The optometrist is well positioned to be alert to both ocular and systemic conditions that may be related both to OSA and CPAP usage and be able to make a timely referral to the patient’s primary care physician or appropriate managing specialist for diagnosis and treatment.
Dr. Kovacich is a clinical associate professor at the Indiana University School of Optometry.
| 1. Santos M, Hofmann J. Ocular manifestations of obstructive sleep apnea. J Clin Sleep Med. 2017;13(11):1345-8. 2. Young T, Skartrud J, Peppard P. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291(16):2013-6. 3. Singh J, Cleveland J. Gout and the risk of incident obstructive sleep apnea in adults 65 years or older: an observational study. J Clin Sleep Med.2018;14(9):1521-7. 4. Karkinski D, Georgievski O, Dzekova-Vidimliski P, et al. Obstructive sleep apnea and lipid abnormalities. Open Access Maced J Med Sci. 2017;5(1):19-22. 5. Davies R. The relationship between neck circumference, radiographic pharyngeal anatomy, and the obstructive sleep apneoa syndrome. European Respiratory Journal. 1990;3(5):509-14. 6. Peppard P, Young T, Barnet, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-14. 7. Dempsey JA, Veasey S, Morgan B, O’Donnell C. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47-112. 8. McNab A. The eye and sleep. Clin Exp Ophthalmol. 2005;33(2):117-25. 9. Gupta P, Stinnett S, Carlson A. Prevalence of sleep apnea in patients with keratoconus. Cornea. 2012;31(6):595-9. 10. Shiba T, Sato Y, Takahashi M. Relationship between diabetic retinopathy and sleep-disordered breathing. Am J Ophthalmol. 2009;147(6):1017-21. 11. Huon LK, Liu SY, Camacho M, Guilleminault C. The association between ophthalmologic diseases and obstructive sleep apnea: a systematic review and meta-analysis. Sleep Breath. 2016;20(4):1145-54. 12. Schlötzer-Schrehardt U, Stojkovic M, Hofmann-Rummelt C, et al. The pathogenesis of floppy eyelid syndrome: involvement of matrix metalloproteinases in elastic fiber degradation. Ophthalmol. 2005;112(4):694-704. 13. Floppy Eyelid Syndrome. In: The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease. 7th ed, Bagheri N, ed. Philadelphia: Wolters Kluwer; 2017:136-7. 14. Perez-Rico C, Gutierrez-Diaz E, Mencia-Gutierrez E, et al. Obstructive sleep apnea-hypopnea syndrome (OSAHS) and glaucomatous optic neuropathy. Graefes Arch Clin Exp Ophthalmol. 2014;252(9):1345-57. 15. Mojon D, Hess C, Goldblum D, et al. Normal-tension glaucoma is associated with sleep apnea syndrome. Ophthalmologica. 2002;216(3):180-4. 16. Mojon D, Hedges T, Ehrenberg B, et al. Association between sleep apnea syndrome and nonarteritic anterior ischemic optic neuropathy. Arch Ophthalmol. 2002;120(5):601-605. 17. Papilledema. In: The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease. 7th ed. Bagheri N, ed. Philadelphia: Wolters Kluwer; 2017:259-60. 18. Jennum P, Borgensen SE. Intracranial pressure and obstructive sleep apnea. Chest. 1989;95(2):279-83. 19. Marcus D, Lynn J, Miller J, et al. Sleep disorders: a risk factor for pseudotumor cerebri? J Neuroophthalmol. 2001;21(2):121-3. 20. Idiopathic Intracranial Hypertension/Pseuodtumor Cerebri. In: The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease. 7th ed, Bagheri N, ed. Philadelphia: Wolters Kluwer;2017:261-2. 21. Javaheri S, Golnik K. Resolution of papilledema associated with OSA treatment. J Clin Sleep Med. 2011;7(4):399-400. 22. Thurtell MJ, Bruce B, Rye D, et al. The Berlin questionnaire screens for obstructive sleep apnea in idiopathic hypertension. J Neuroophthalmol. 2011;31(4):316-9. 23. Central retinal vein occlusion. In: The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease. 7th ed, Bagheri N, ed. Philadelphia: Wolters Kluwer;2017:289-91. 24. Ceriello A. New insights on oxidative stress and diabetic complications may lead to a “causal” antioxidant therapy. Diabetic Care. 2003;26(5):1589-96. 25. Mohamed Q, Gillies M, Wong T. Management of diabetic retinopathy: A systematic review. JAMA. 2007;298(8):902–16. 26. Schulz R, Hummel C, Heinemann S, et al. Serum levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea and severe nighttime hypoxia. Am J Respir Crit Care Med. 2002;165(1):67-70. 27. Diabetic retinopathy. In: The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease. 7th ed, Bagheri N, ed. Philadelphia: Wolters Kluwer;2017:295-300. 28. Central Serous Chorioretinopathy. In: The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease. 7th ed, Bagheri N, ed. Philadelphia: Wolters Kluwer; 2017:305-6. 29. Mitchell R, Hussey H, Setzen G, et al. Clinical consensus statement: tracheostomy care. Otolarynol Head Neck Surg. 2013;148 (1):6-20. 30. Weaver T, Grunstein R. Adherence to continuous positive airway pressure therapy. Proc Am Thorac Soc. 2008;5(2):173-8. 31. Giles T, Lasserson T, Smith B, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database of Sys Rev. 2006;25(1):CD001106. 32. Donovan L, Boeder S, Malhotra A, Patel S. New developments in the use of positive airway pressure for obstructive sleep apnea. J Thorac Dis. 2015;7(8):1323-42. 33. Bucks R, Olaithe M, Eastwood P. Neurocognitive function in obstructive sleep apnoea: A meta-review. Respirology. 2013;18(1):61-70. 34. Olaithe M, Bucks R. Executive dysfunction in OSA before and after treatment. A meta-analysis. Sleep. 2013;36(9):1297-305. 35. Araghi MH et al. Effectiveness of lifestyle interventions on obstructive sleep apnea (OSA): systematic review and meta-analysis. Sleep. 2013;36(10):1553-62. 36. Stauffer J, Fayter N, MacLurg B. Conjunctivitis from nasal CPAP apparatus. Chest. 1984;86(5):802. 37. Harrison W, Pence N, Kovacich S. Anterior segment complications secondary to continuous positive airway pressure machine treatment in patients with obstructive sleep apnea. Optometry. 2007:78(7);352-55. 38. Singh N, Walker R, Cowan F, et al. Retrograde air escape via the nasolacrimal system: a previously unrecognized complication of continuous positive airway pressure in the management of obstructive sleep apnea. Ann Otol Rhinol Laryngol. 2014;123(5):321-4. 39. Cannon P, Madge S, Selva D. Air regurgitation in patients on continuous positive airway pressure (CPAP) therapy following dacrocystorhinostomy with or without Lester-Jones tube insertion. Br J Ophthalmol. 2010;94(7):891-3. 40. Goktas O, Haberman A, Thelen A, Schrom T. The punctum plug as an option for treating retrograde airflow from the lacrimal sac. Laryngorhinootologie. 2007;86(10):732-5. 41. Kiekens S, De Groot V, Coeckelbergh T, et al. Continuous positive airway pressure therapy is associated with an increase in intraocular pressure in obstructive sleep apnea. Invest Ophthalmol Vis Sci. 2008;49(3):934-40. |

