 Ideally, our circulatory system operates like a well-oiled machine, with all the vessels and arteries making seamless deliveries of blood throughout the body. When the lines of this complex system get tangled and arteries connect directly to veins with no capillary bed to cushion them, however, it can cause arteriovenous malformations—and if those AVMs occur in the brain or spine, the impact on general and ocular health can be considerable.
Ideally, our circulatory system operates like a well-oiled machine, with all the vessels and arteries making seamless deliveries of blood throughout the body. When the lines of this complex system get tangled and arteries connect directly to veins with no capillary bed to cushion them, however, it can cause arteriovenous malformations—and if those AVMs occur in the brain or spine, the impact on general and ocular health can be considerable.
This defect of the circulatory system is thought to arise during embryonic or fetal development, or soon after birth. AVMs can occur in various parts of the body with little health risk or symptoms, but neurological AVMs of the brain or spinal cord can cause cerebral bleeding, seizures and optic disc edema secondary to intracranial hypertension.
The Facts on AVMs
Neurological AVMs affect about 300,000 Americans. Although AVM occurs in both sexes and all ethnicities equally, it is most commonly seen in young adults, with morbidity occurring in 30% to 50% of patients and death rates of 10% to 15%.1 Cerebral bleeding and seizures are the most typical modes of presentation.
AVMs produce neurological dysfunction via three primary mechanisms. First, through hemorrhage. This most commonly occurs in the brain parenchyma, but can be found in the subarachnoid or intraventricular space. Hemorrhage from cerebral AVMs represents 2% of all hemorrhagic strokes.2 In the absence of hemorrhage: seizures. About 15% to 40% of patients present with a seizure disorder.
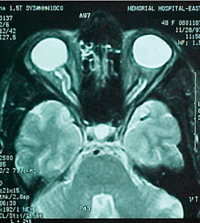
| |
|
T2 Axial cut shows partially empty sella.
|
The third manifestation of arteriovenous malformations is a progressive neurological deficit, which occurs in 6% to 12% of AVM patients over a span of months or years. This process, known as the “steal phenomenon,” siphons blood away from adjacent brain tissue.1,2
Notably, a growing number of asymptomatic AVMs have been encountered due to increased MRI use. The clinical course of these cases is mild in comparison to those that present with symptomatic cerebral hemorrhage.1,2
Diagnostic Work-up
It’s critical to always perform careful evaluations of personal and familial history in cases of suspected systemic disease, and arteriovenous malformations are no exception. It’s important to note that AVMs may be associated with other inherited disorders, such as Sturge-Weber disease, neurofibromatosis and von Hippel-Lindau syndrome, a rare genetic disorder whose clinical hallmarks include the development of retinal and central nervous system blood vessel tumors.1,2
AVMs have a tendency to lurk silently. It’s common for the condition to remain undetected until the presenting event, which means the diagnosis is usually made at the time of the first seizure or hemorrhage.
Although exact diagnostic symptoms are difficult to pinpoint, a history of headaches has been found in as many as half of all patients with cerebral arteriovenous malformation. Risk factors—be they genetic, demographic or environmental—have not been clearly identified, further complicating diagnostics.
| ARUBA |
|
The Randomized trial of Unruptured Brain Arteriovenous
malformations—better known as the ARUBA study—is a clinical trial
sponsored by the National Institutes of Health. ARUBA was designed to
discover best practices for caring for those with a neurological
arteriovenous malformation (AVM) that has never bled.
ARUBA found that medical management lowered the risk of death or stroke in patients with unruptured AVMs at more promising rates than medical management coupled with interventional therapy, such as neurosurgery, embolization and/or stereotactic radiotherapy. Although the trial is continuing its observational phase, the results were so favorable to exclusive medical management after 33 months that a data and safety monitoring board halted the randomization. The trial will continue observation for five years post follow-up.4 |
It is especially important for the optometrist to note symptoms of transient visual obscurations and recurrent headaches, should they exist. Proper ophthalmic workup includes visual acuity testing, along with eye movement evaluation, pupils, confrontation and Amsler Grid testing.
A comprehensive ocular health evaluation, including dilated fundus evaluation, is essential. Ancillary testing, such as color vision, central threshold perimetry, optical coherence tomography, and ocular ultrasound are often useful in such cases.
Neuroimaging
Proper diagnosis of AVM rests on the shoulders of high-quality radiologic imaging.
| S econdary Psuedotumor Syndromes5 | |
|
Venous sinus thrombosis
Arteriovenous malformation Hypertension Congestive heart failure Guillian-Barre Medications (vitamin A, tetracycline, minocycline, lithium, penicillin, oral contraception) Tumors Obesity and weight gain Hypercoagulability Anemia Systemic Lupus Sleep apnea Head trauma |
AVMs appear as irregular or globoid masses within the hemispheres or the brain stem. The mass might be surrounded by, or inclusive of, a low signal of extracellular hemosiderin, indicative of prior hemorrhage.
While CT imaging can only identify relatively large AVMs, it can easily pinpoint intracerebral hemorrhage, which should alert clinicians to potential AVM—particularly among younger patients. MRI is essential for initial diagnosis.
Cerebral angiography is required for hemodynamic assessment, which is essential to charting a course of treatment.
The treatment plan will depend on the morphology of the AVM, including features of the feeding arteries, arterial and venous aneurysms and venous drainage patterns.3
Treatment Approaches
The appraoch to treatment of arteriovenous malformation is dependent on several factors, most notably the risk of initial or subsequent hemorrhage. This risk is typically determined by the patient’s demographic, historical and angiographic features. Smaller AVM size, deep venous drainage and high arterial feeding pressures increase the likelihood of subsequent hemorrhage.1
Invasive treatment—including endovascular embolization, surgical resection and focal beam radiation—is recommended for younger patients with one or more high-risk features. These treatments can be used alone or in combination.1,2
For older individuals and low-risk patients, anticonvulsants and analgesia for headaches may be the only necessary treatment. Common medications include phenytoin, carbamazepine, valproic acid and lamotrigine.1 No specific activity restrictions are placed on patients with AVM.
A multispecialty approach should be taken with AVM patients, with eye care providers playing an important role among the neuro team, which typically includes a neurologist, neuropsychologist, neurosurgeon, interventional neuroradiologist and neuroanesthesiologist.
Patients frequently present to the optometric physician with symptoms of headache and transient blur. Often, these symptoms prove to arise from non-urgent causes. However, it is crucial for the practitioner to rule out sight-threatening and sometimes life-threatening causes during the encounter. Interrogate and investigate for telltale ocular symptoms and clinical signs, particularly those that may relate to the pupillary and visual pathways, as well as cranial nerves.
Bilateral disc edema may occur due to several conditions. It is important to rule out neoplastic disease, as well as intracranial hypertension due to a variety of causes, including cerebral AVMs.
|
Case Report
|
|
• History. A 48-year-old, non-obese white female presented recently to
the clinic with symptoms of transient visual obscurations in each eye
and recurrent headaches. Systemic history was positive for a resolved
intracranial vascular malformation diagnosed eight years prior.
• Diagnostic data. Dilated fundus examination revealed asymmetric optic disc edema (OS > OD). B-scan ultrasonography characterized the resulting asymmetric ONH elevation. Central 24-2 visual field test results showed inferonasal defects OD and scattered edge defects OD and OS. MRI with and without contrast confirmed the previous vascular abnormality in the left parietal lobe. A neuroradiologist reported radiologic features consistent with AVM. Axial and sagittal MR showed a decrease in the signal encircling the AVM, due to hemosiderin from leaked blood. • Management. Lumbar puncture revealed an opening pressure of 320mm H20. This established a diagnosis of intracranial hypertension secondary to AVM. Diamox 500mg BID was prescribed. Due to intolerable adverse reactions, this medication was switched to Lasix 20mg BID with KCl 20mEq BID. The patient is receiving ongoing care by a multispecialty team, with invasive treatment the likely next step. |
1. Novakovic RL, Lazzaro MA, Castonguay AC, Zaidat OO. The diagnosis and management of brain arteriovenous malformations. Neurol Clin. 2013 Aug;31(3):749-63.
2. Laakso A, Dashti R, Juvela S, et al. Natural history of arteriovenous malformations: presentation, risk of hemorrhage and mortality. Acta Neurochir Suppl. 2010;107:65-9.
3. Halim AX, Johnston SC, Singh V. Longitudinal risk of intracranial hemorrhage in patients with arteriovenous malformation of the brain within a defined population. Stroke. 2004 Jul;35(7):1697-702.
4. Mohr JP, Parides MK, Stapf C, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014 Feb 15;383(9917):614-21.
5. Perez MA, Glaser, JS Shatz NJ. Idiopathic intracranial hypertension caused by venous sinus thrombosis associated with contraceptive usage. Optometry. 2010 Jul;81(7):351-8.
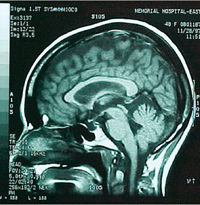
|
|
|
Partially empty sella visible on T1 sagittal view.
|

