Lucentis (ranibizumab, Genentech) can stop growth and leakage from choroidal neovascularization (CNV) and help to restore vision in patients who have age-related macular degeneration, according to two clinical studies in the October 5 New England Journal of Medicine.1,2
In the Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular Age-Related Macular Degeneration, or MARINA, researchers randomized 716 patients to receive 0.3mg or 0.5mg of ranibizumab or a sham injection for two years.
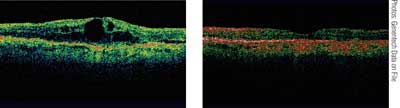 |
| Optical coherence tomography images show the same patient at baseline (left) and |
At 12 months, 95% of patients in each Lucentis group lost fewer than 15 letters of visual acuity (VA) vs. 62% of control subjects. In fact, VA improved by a mean 6.5 letters and 7.2 letters in the 0.3mg and 0.5mg groups, respectively, but decreased by a mean 10.4 letters in the control group. Also, 40% of patients receiving Lucentis had VA of 20/40 or better at 12 months vs. 11.3% of the control group.
By 24 months, 90% or more patients in each Lucentis group lost fewer than 15 letters vs. 52.9% of control subjects. Some 35% and 42% of subjects in the 0.3mg and 0.5mg groups, respectively, achieved VA of at least 20/40 vs. 6% of subjects in the control group.
At 12 and 24 months, 25% of patients in the 0.3mg group and 33% of patients in the 0.5mg group gained 15 or more letters of visual acuity vs. 5% or less in the control group.
In the second study, the Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age-Related Macular Degeneration (ANCHOR) trial, researchers randomized 423 patients to receive 0.3 or 0.5mg of ranibizumab plus sham photodynamic therapy (PDT) with Visudyne (verteporfin, Novartis Ophthalmics) or sham intravitreal injections plus active PDT for 12 months.
At 12 months, 94% and 96% of patients in the 0.3mg and 0.5mg groups, respectively, lost fewer than 15 letters of VA vs. 64% in the verteporfin group. VA improved by 15 or more letters in about 38% and 40% of the two groups, respectively, and by about 6% in the verteporfin group. Some 13% of control subjects lost 30 or more letters of VA, but no patients using Lucentis had vision loss this severe.
Some 7% of patients in the 0.3mg group and 6% in the 0.5mg group achieved VA of 20/20 or better at 12 months vs. fewer than 1% of patients in the verteporfin group. Patients in the 0.3mg and 0.5mg groups achieved increased VA of 8.5 and 11.3 letters, respectively, while patients in the control group lost a mean 9.5 letters.
Both studies were supported by Genentech and Novartis Pharmaceuticals.
Lucentis, which received FDA approval on June 30, binds to and inhibits all biologically active forms of vascular endothelial growth factor (VEGF)-A, which causes CNV. The cost of Lucentis, however, is about $1,950 per 0.5mg dose.3 So, eye-care providers have used Genentechs metastatic colon cancer drug Avastin (bevacizumab) off-label to treat CNVat an estimated cost of $17 to $50 per injection.3 (See A Rivalry Between Two AMD Drugs, October 15, 2006.)
Although both drugs are similar in their mechanism of action, Genentech has argued unapproved ocular use of Avastin puts patients at risk for complications.
A head-to-head study of ranibizumab and bevacizumab and a careful evaluation of an induction and follow-up strategy with either drug are probably the next most useful steps in this field, writes Edwin M. Stone, M.D., Ph.D., in the same issue of NEJM.4
To that end, the National Eye Institute has recently approved and funded a trial to compare Lucentis to Avastin in eligible patients with wet AMD. Patient enrollment is expected to begin after January 1.
1. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006 Oct 5;355(14):1419-31.
2. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006 Oct 5;355(14):1432-44.
3. Steinbrook R. The price of sight-ranibizumab, bevacizumab, and the treatment of macular degeneration. N Engl J Med 2006 Oct 5;355(14):1409-12.
4. Stone EM. A very effective treatment for neovascular macular degeneration. N Engl J Med 2006 Oct 5;355(14):1493-5.

