Level Up Your TechOptometrists today have more tools than ever at their disposal to help detect, treat and manage disease. To help you keep up with the constant innovation, the September issue of Review of Optometry—our 46th annual technology report—gives readers a rundown on what's new in FAF imaging, visual field testing, ultra-widefield imaging, low vision and more. |
Retinal imaging has evolved significantly, from the first black and white picture of the retina captured in 1886 to 40 years later when the first commercially available camera was developed by Carl Zeiss in 1926 with a 20° field of view.1 By the end of 20th century, retinal cameras could routinely capture a 45° image of the posterior pole; with montaging of multiple photographs, a 96° view of the fundus was possible (Figure 1).2
Today, modern widefield (WF) and ultra-widefield (UWF) imaging devices provide digital images that have the capability to capture over 200° of the retina. These cameras are very useful for screening, diagnosing and managing a wide array of retinal diseases. Many peripheral imaging systems now offer features such as color fundus photography, fluorescein angiography (FA), indocyanine green angiography, fundus autofluorescence (FAF) and spectral domain or swept-source OCT.
UWF Imaging Definitions
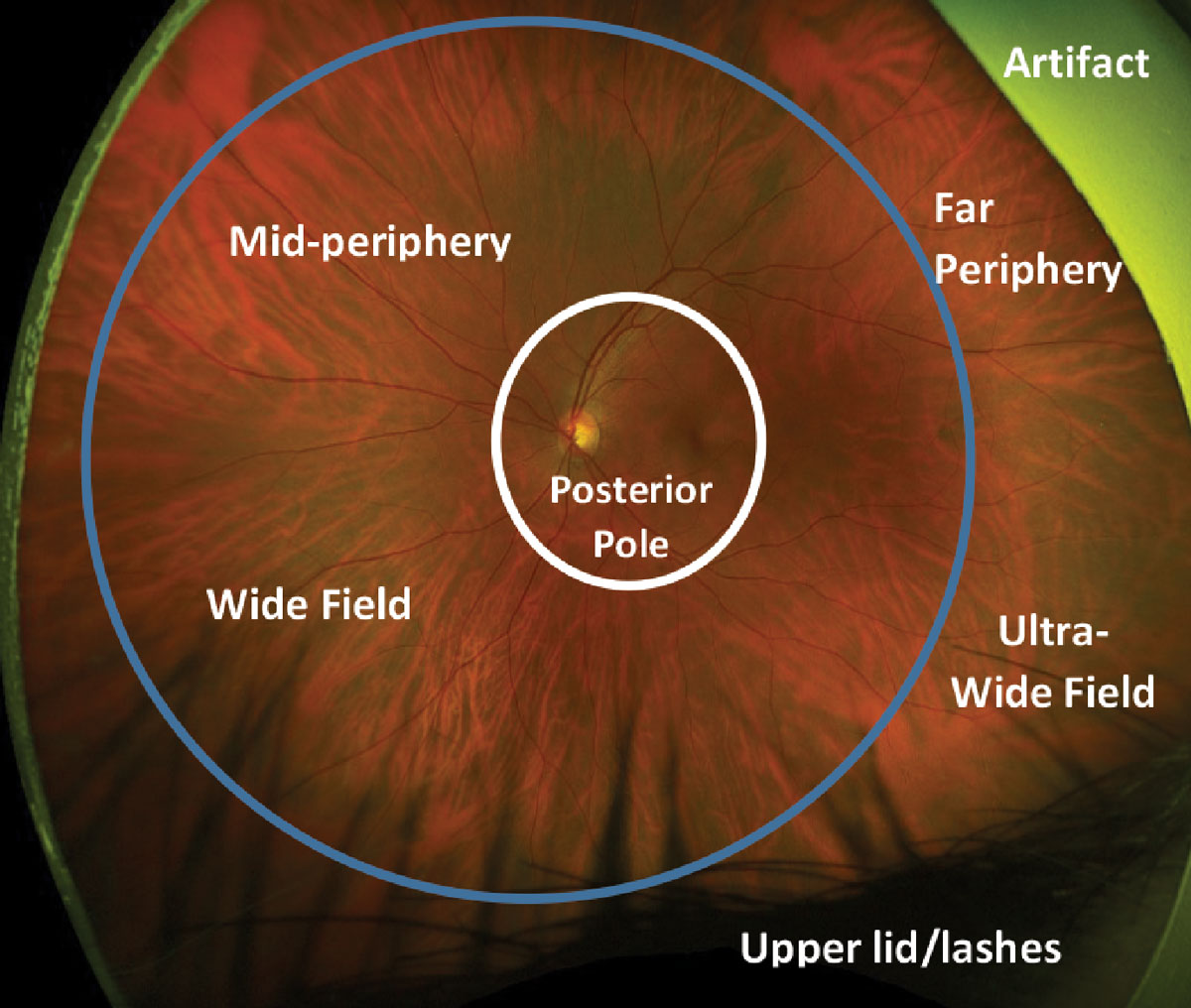
These were formalized for various fundus imaging modalities in 2019 and are based on anatomic landmarks.3 The posterior pole is defined as the central 50° of the retina, which extends just beyond the vascular arcades. WF photography is defined as images with a field of view of approximately 60° to 100°, capturing the mid-peripheral retina from the anterior aspect of the vascular arcades to the posterior edge of the vortex vein ampullae. UWF imaging captures the far peripheral fundus, which includes the retina from the anterior edge of the vortex vein ampulla to the pars plana with a 110° to 220° field of view (Figure 2). The term panretinal describes an image that includes the entire retina to the ora serrata 360° around.
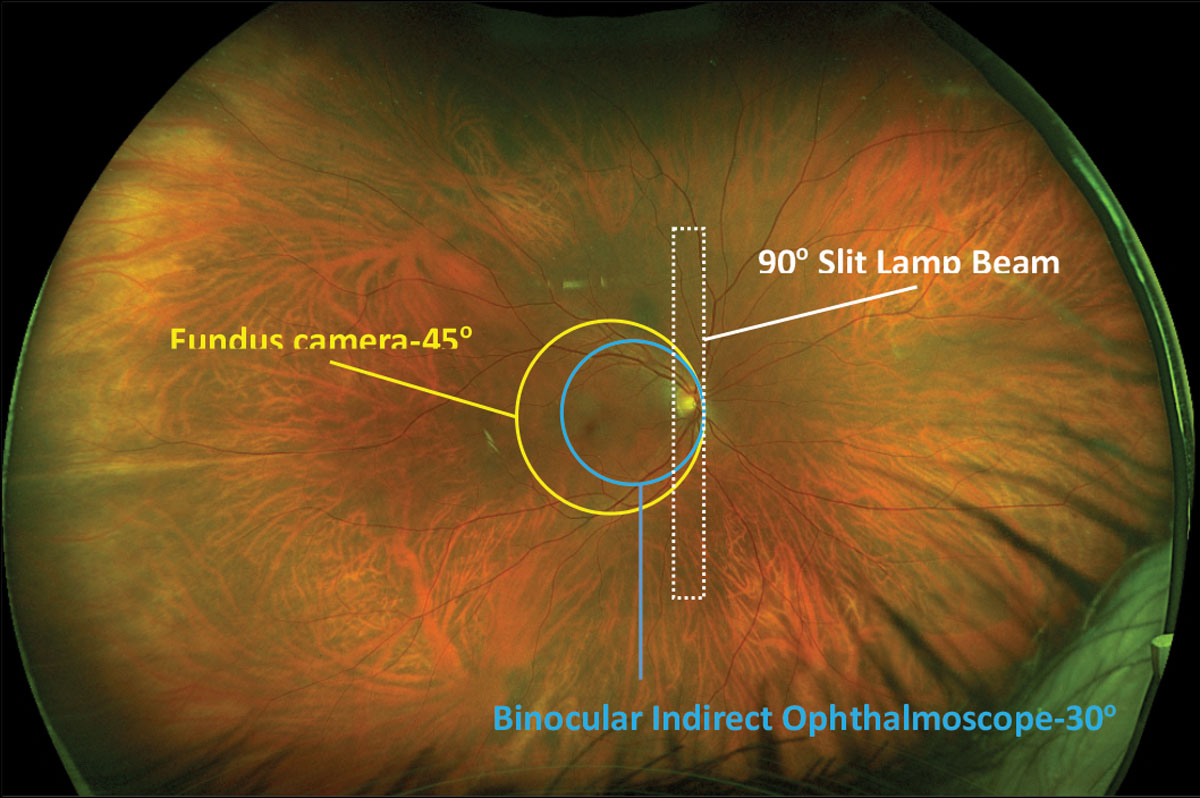 |
Fig. 1. Comparisons of fields of view from different fundus imaging devices overlaid on an Optos photo. Click image to enlarge. |
Types of Cameras
There are several widefield instruments currently in use, including the following: Optos (Optos), Heidelberg Spectralis, (Heidelberg Engineering), Clarus 700 (Carl Zeiss Meditec), Eidon (CenterVue) and RetCam (Clarity Medical Systems).
UWF imaging systems that use confocal laser ophthalmoscopy include Optos, Heidelberg Spectralis and Eidon. Optos combines a confocal scanning laser ophthalmoscope (cSLO) with an ellipsoid mirror and is capable of capturing 200° images (approximately 82%) of the retina in less than half a second with 11µm to 14µm/pixel resolution.1 Using the auto-montage feature on the machine, which combines up, down, left and right gaze steered images, Optos can photograph 220° (approximately 97%) of the retina.4 Optos uses low-powered red and green lasers to produce pseudocolor images, blue laser for FA, green laser for FAF and infrared laser for indocyanine green angiography.
Heidelberg Spectralis also uses cSLO imaging and is capable of taking UWF photos with a resolution of 10µm/pixel.1 The Heidelberg system can take non-contact images up to 105° (with the HRA 2 model) with considerably less lash artifacts compared to Optos. With the addition of a contact Staurenghi lens (Ocular Staurenghi 230 SLO Retina Lens, Ocular Instruments), the Heidelberg system can produce a 150° image of the retina.
The main disadvantages of an imaging system that requires contact with the ocular surface are the need for a skilled photographer and a highly cooperative patient. The Eidon system uses cSLO and white LED light to take a 90° true color photo in a single capture and can image up to 160° with a montage of multiple photographs.5
Other ultrawide imaging systems available are the Clarus 700 and RetCam. Clarus is a non-contact camera with partially confocal optics that reduces lash and lid artifacts. This camera provides true color images with a resolution of 7µm/pixel, capturing 133° in a single image and 200° with two combined images.1 RetCam also has a similar 130° field of view and is most commonly used in the pediatric population. It’s portable and can take images in the supine position, which is advantageous for newborns and bedridden adults, but does require dilation.
 |
|
Fig. 2. Defining widefield and ultra-widefield on an Optos photo. The white circle represents the posterior pole of the fundus. The retina inside the blue circle represents widefield imaging and mid peripheral retina. The retina outside the blue circle represents ultra-widefield imaging and the far periphery. Click image to enlarge. |
Avoiding Artifacts
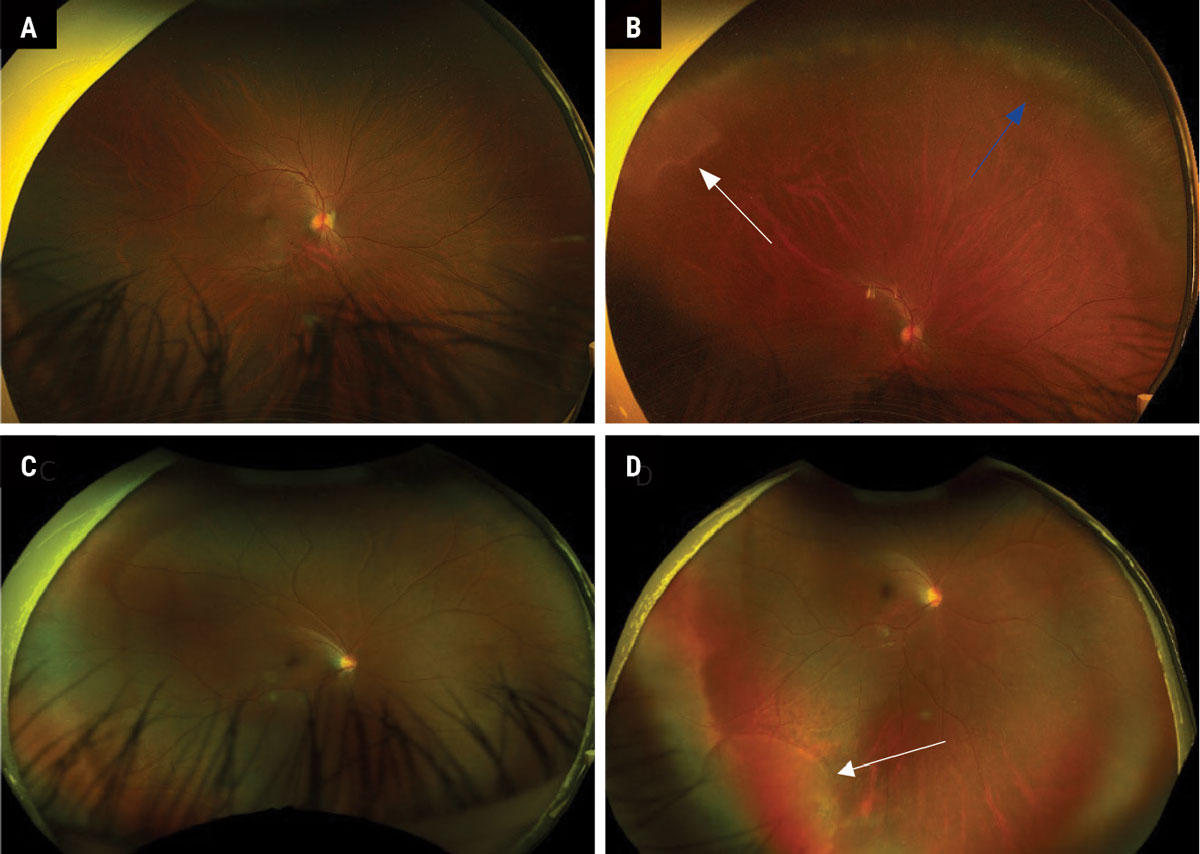
Proper patient and camera positioning is key to capturing any good quality retinal image. Far peripheral lesions can be difficult to image, even for experienced ophthalmic photographers. Having the patient look in the direction of the lesion and using the “steering” feature on the camera (if available) often allows for the peripheral lesion to come into view and will provide an improved quality photo (Figure 3). It may also be helpful to have the clinician present when taking a peripheral image to help the technician locate the area in question.
The patient can also help align themselves for optimal results by moving their head in or out depending on the location and/or the color of the target that they see in the instrument. Common artifacts in UWF imaging occur from the eyelashes, eyelids and nose. To minimize the amount of lid and lash artifacts in an image, ask the patient to open their eye as wide as possible. Lifting the patient’s eyebrow or raising the height of the table may also reduce upper eyelid artifacts that appear at the bottom of the photo. With some imaging systems, such as the Optos, the patient’s head needs to be turned so that the nose remains out of the picture.
Often, it is helpful to tell the patient to position their head as if they were looking through a keyhole in a door, as poor head positioning can lead to a bright reflex of light that can be mistaken for a retinal lesion. Vitreous floaters often cast shadows that masquerade as dark lesions on the retina, mimicking conditions such as blood or a choroidal nevus. Examining additional UWF images of a presumed lesion for a change in position on the retina is beneficial in identifying such an anomaly. As with any potential artifact found on photography, suspicious findings should be confirmed with a dilated eye exam.
 |
|
Fig. 3. (A) Normal-appearing Optos fundus photo in straight-ahead gaze. (B) Steered photo of patient A, looking up to reveal peripheral cystoid degeneration (blue arrow) and white without pressure (white arrow). (C) Normal-appearing Optos fundus photo in straight-ahead gaze. (D) Steered photo of patient C, looking down and out to reveal a shallow inferior temporal retinal detachment (white arrow). Click image to enlarge. |
UWF Imaging vs. Dilation
While WF imaging has come a long way, there are no retinal imaging systems currently capable of taking a panretinal photograph and capturing an image of the entire retina in a single click of a button. Therefore, UWF imaging does not replace a dilated fundus exam, but can certainly enhance the ability to locate and document retinal abnormalities. Small lesions that are seen with binocular indirect ophthalmoscopy can be photographed and then digitally magnified to aid in differential diagnosis.
Advantages to UWF imaging are that it is safe, fast, painless and does not require mydriasis. However, it can miss subtle retinal pathology, especially beyond 200° in the far periphery. Pseudo coloration and magnification distortions of images can allow retinal findings to go undiagnosed in the absence of a dilated fundus exam. Imaging systems that use cSLO technology combine monochromatic red and green light rather than full color spectrum white light. The two-tone, semi-realistic coloration of the fundus produced by scanning ophthalmoscopes makes detecting subtle retinal changes more difficult.
Magnification distortions occur when a three-dimensional image is placed onto a two-dimensional flat surface. Projecting a curved surface like the retina onto a flat screen can lead to peripheral lesions appearing larger than if they were located more centrally. An example of this phenomenon would be how a two-dimensional map representing our three-dimensional world distorts the size of land masses near the poles, known as the “Greenland effect.”6 Fortunately, there is stereoscopic projection software incorporated into some UWF imaging systems that attempts to remedy these peripheral distortions.1,2
 |
|
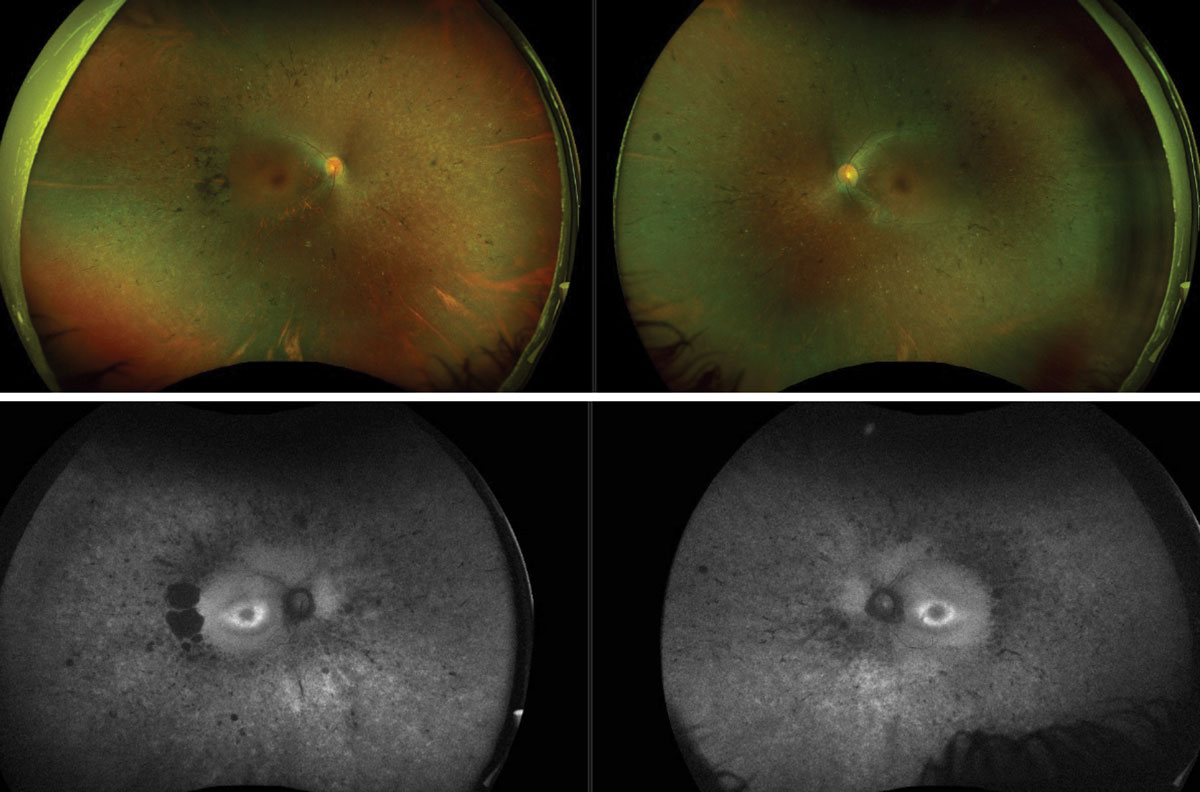
Fig. 4. Color Optos photos of retinitis pigmentosa (top). FAF Optos images of the same patient (bottom). Click image to enlarge. |
Diagnosis and Management of Disease
UWF imaging has led to a better understanding of the peripheral retina and its role in a wide array of retinal vascular conditions and inherited retinal diseases. With inherited retinal diseases, UWF imaging is excellent for documenting the level of involvement in nearly the entire retina. In these patients, UWF-FAF can be invaluable in helping to differentially diagnose various disorders (Figure 4).
Retinal conditions with areas of peripheral capillary nonperfusion such as diabetic retinopathy, retinal vein occlusion (RVO), sickle cell retinopathy and ocular ischemic syndrome can be more effectively diagnosed and managed with UWF images, particularly incorporating UWF angiography.2,6 UWF imaging can also be very helpful in diagnosing posterior uveitis, retinal vasculitis and posterior ocular tumors. Research also continues to study the clinical significance of the peripheral retina in macular conditions such as age-related macular degeneration and edema associated with DR and RVO.
Diabetic Retinopathy
Traditional diagnosis, classification and management of DR has centered on vascular changes seen in the posterior pole, not fully considering the role peripheral nonperfusion and ischemia play in the overall progression of the disease. The gold standard for the assessment and grading of DR severity has been the seven-standard field (SSF) photographs as defined by the Early Treatment Diabetic Retinopathy Study (ETDRS). However, SSF imaging can only capture about 75° of the fundus with a montage of seven 30° photos, which encompasses approximately 34% of the retina (Figure 5).2,6 By contrast, UWF imaging can capture over 80% of the retina and better assess the full extent of nonperfusion.
UWF has been found to be at least as sensitive (84% to 94%) and specific (90% to 100%) as the ETDRS SSF montage images for screening and staging DR.6,8-10 In fact, studies show that 40% of DR-related changes occur outside the conventional SSF field of view, resulting in higher levels of DR detected in 9% to 15% of eyes imaged with UWF technology.6,11-15
Using ultra-widefield fluorescein angiography is useful for identifying, quantifying and monitoring areas of peripheral capillary nonperfusion and ischemia in retinal vascular diseases. Research has found DR that is predominately located in the periphery to be four times more likely to progress to proliferative DR over a four-year period.16-18 In fact, when DR was located mainly in the periphery, UWF-FA was found to be better at predicting future DR worsening than UWF color photos alone.18-20 Another study using UWF-FA found that proliferative DR developed with a threshold of 118 disc areas of retinal capillary nonperfusion.6-21 UWF-FA also allows clinicians to follow areas of retinal nonperfusion and target laser photocoagulation treatment if necessary.22-24
UWF-OCT is also available and capable of producing fast, high-resolution, dye-free angiographic images. The parameters of this modality are not solidified in the literature. In general, experts in the field consider OCT to be UWF when the field of vision is equal to or greater than 90°, which can only be reached using a composite of several pictures.25,26 One drawback to imaging the retina with OCT is a much smaller field of view compared to dye-based angiography. An OCT field of 12mm x 12mm covers 50° of the retina compared to UWF-FA which can photograph 110° to 220° in a single image.27
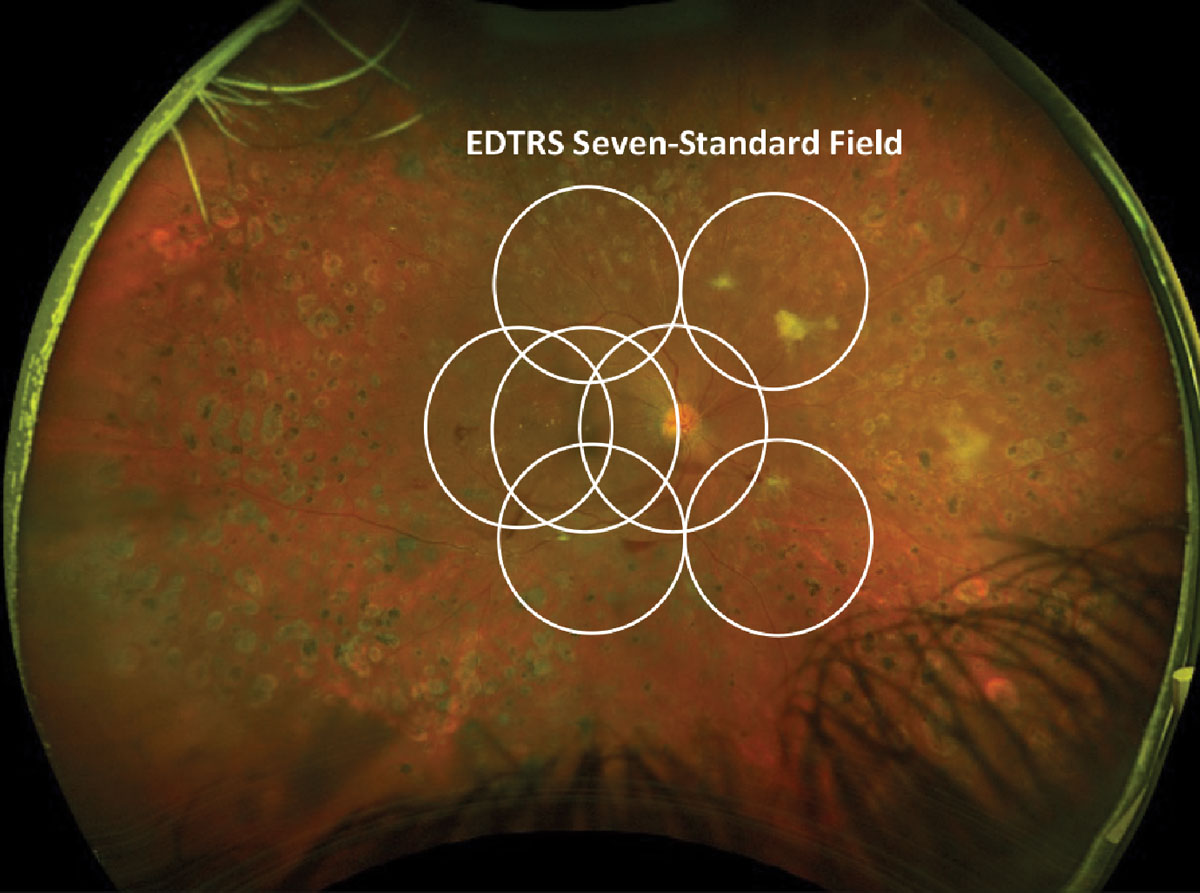 |
Fig. 5. EDTRS seven-standard field images laid over an Optos proliferative diabetic retinopathy photograph. Click image to enlarge. |
RVO
This is another vascular disorder where areas of peripheral retinal nonperfusion extensively contribute to the disease process (Figure 6). Capillary dropout and ischemia are believed to cause the upregulation of pro-inflammatory cytokines such as vascular endothelial growth factor (VEGF). Presumably, larger areas of non-perfused retina extending into the periphery are thought to be associated with elevated levels of intraocular VEGF.28,29
Capillary nonperfusion can be graded using an ischemic index or judging the anatomical extent (in mm2) of the involved retina. The ischemic index is a ratio that compares the number of pixels within the area of capillary nonperfusion to the total number of pixels within the entire visible retina.30 In contrast, judging the anatomical extent of nonperfusion in mm2 often identifies a larger area of nonperfusion.6
Although there is no strict definition, ischemic RVO has often historically been defined as greater than 10 disc areas of nonperfusion on SSF-FA.1,31 With continued adoption of UWF-FA and OCT-A imaging, definitions of peripheral nonperfusion are rapidly evolving. Research using ultra-widefield FA suggests that most nonperfusion in a central RVO occurs in the temporal retina and that a total of 30 disc areas of nonperfusion—rather than 10—may be a better threshold for considering a central RVO to be ischemic.32,33 Research with widefield OCT-A suggests defining ischemic RVO as having >30% decreased flow area throughout the entire extent of the imaged retina.26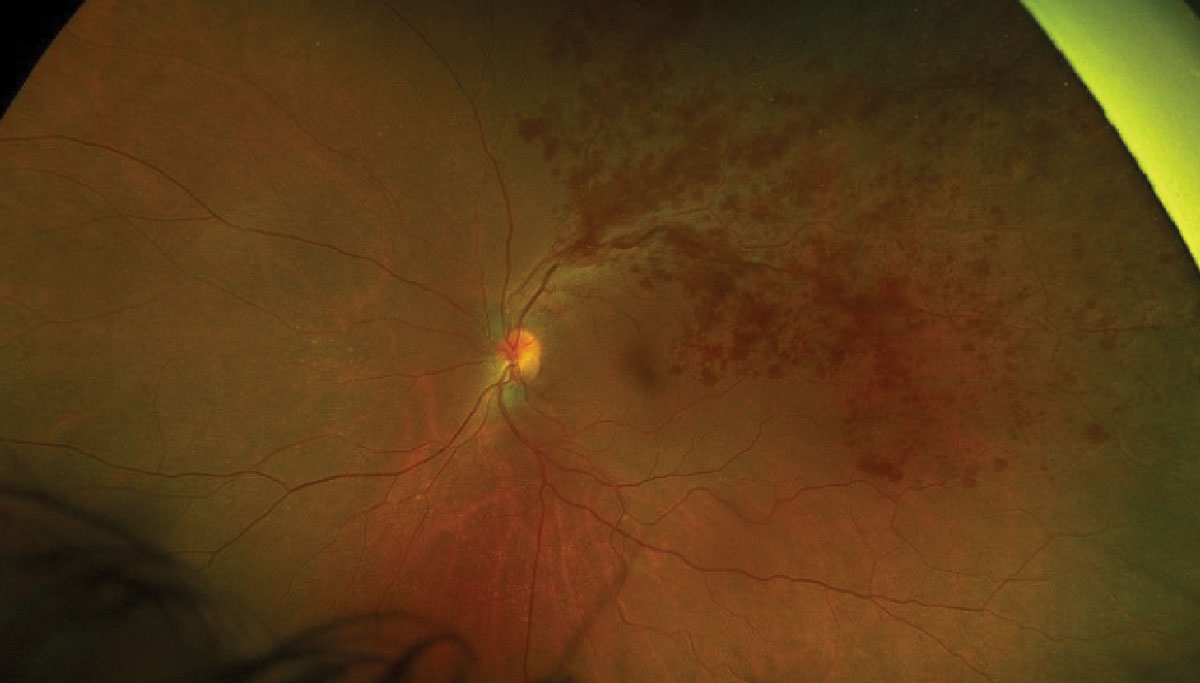 |
|
Fig. 6. Optos photo of a large branch retinal vein occlusion. Click image to enlarge. |
Far Peripheral Lesions
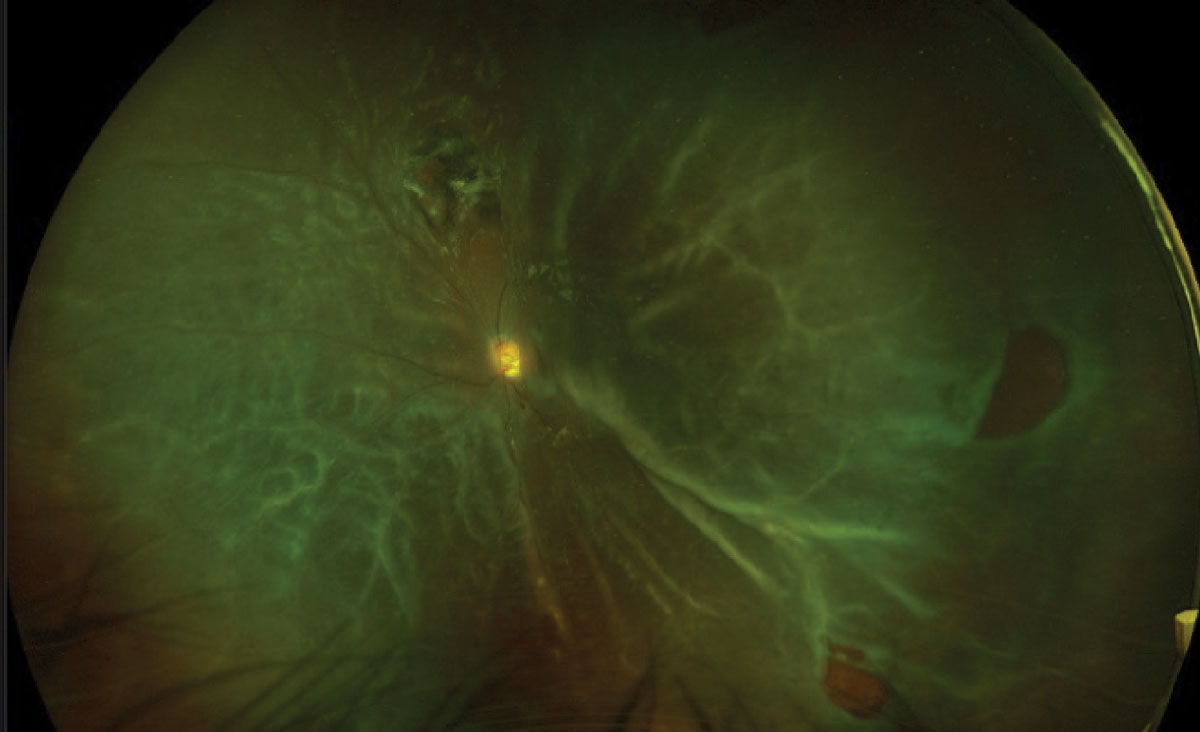
UWF photography allows for imaging a host of peripheral retina lesions such as retinal detachments, holes, tears, lattice degeneration and retinoschisis. For example, imaging can have a role in detecting and managing rhegmatogenous retinal detachment (RRD). The ability to image the rhegmatogenous detachment allows for better patient education, surgical planning and monitoring of the condition (Figure 7). Also, UWF-FAF imaging may reveal a more accurate demarcation line, thus better evaluating the extent of the RRD.34
Obtaining a cross-sectional view of far peripheral lesions using UWF-OCT can provide detailed information on the extent of vitreal traction, subretinal fluid, relative retinal thinning, attenuation of choroidal vessels and cystoid degeneration of peripheral pathologies.35
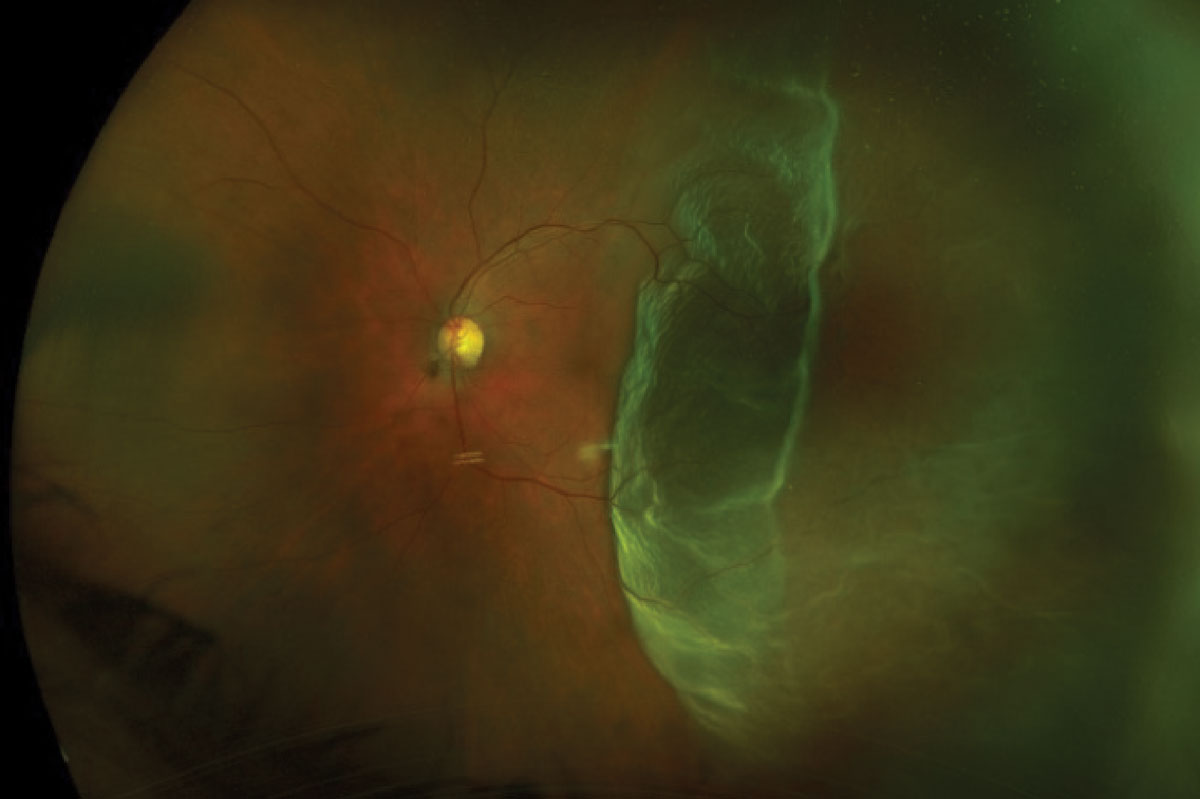 |
Fig. 7. Optos photo of a macula-off retinal detachment (top). Optos photo of a total retinal detachment (bottom). Click images to enlarge. |
 |
Pediatrics
Diagnosing and managing vitreoretinal disorders in the pediatric population can be challenging and often requires exams to be done under sedation with contact-based imaging systems. The RetCam has been used for many years in the neonatal population to screen for retinopathy of prematurity.36 This camera is especially useful because it can perform FA on these children, which aids in the diagnosis and management of this and other peripheral retinal diseases.37 Other contact-based imaging systems used in the pediatric population include Heidelberg Spectralis, PanoCam LT (Visunex Medical Systems) and 3nethra Classic (Forus Health).2
Capturing non-contact retinal images on neonates is difficult but possible by holding the newborn in the “flying baby” position.6 With this technique, the infant is held face down on the parent’s inner forearm, supporting the chest and chin, while the other hand supports the baby’s head. The newborn is then held up to the camera and positioned properly by the parent to allow a technician to capture an image.
Takeaways
When used properly, UWF imaging does not routinely replace dilation, but instead augments our ability to fully evaluate the peripheral fundus. It has brought new insights into the importance of the peripheral retina in various retinal disorders. Not only can UWF imaging allow detailed documentation of peripheral retinal findings, it can also improve diagnosis, staging classifications, monitoring and management of many common retinal conditions. The future of UWF imaging will likely include smartphone-based, portable devices to virtually screen the retina and the development of artificial intelligence-based software to improve the analysis of peripheral retinal pathology.37-40
As UWF imaging technology evolves, expanding our field of view, we will likely continue to gain new insights into the role the peripheral fundus plays in ocular disease.
Dr. Sutton is a clinical professor at Indiana University School of Optometry and Service Chief of the Indianapolis Eye Care Center. He is a Fellow of the American Academy of Optometry, the Optometric Retina Society and the Optometric Glaucoma Society, as well as a member of the American Optometric Association. Dr. Torbit is an associate clinical professor at Indiana University School of Optometry. She is a Fellow of the American Academy of Optometry, the Optometric Retina Society and the Optometric Glaucoma Society. They have no financial disclosures.
1. Haner NU, Dysli C, Muck MR. Imaging in retinal vascular disease: A review. Clin Experiment Ophthalmol. 2023;51:217-28. 2. Patel SN, Shi A, Wibbelsman TD, Klufas MA. Ultra-widefield retinal imaging: an update on recent advances. Ther Adv Ophthalmol. 2020;(12)1-12. 3. Choudhry N, Duker JS, Freund KB, et al. Classification and guidelines for widefield imaging: recommendations from the International Widefield Imaging Study Group. Ophthalmol Retina. 2019;3:10:843-9. 4. Calvo CM., Hartnett ME. The utility of ultra-widefield fluorescein angiography in pediatric retinal diseases. Int J Retin Vitr. 2018;4(21). 5. Midena E., Marchione G, Digiorgio S, et al. Ultra-wide-field fundus photography compared to ophthalmoscopy in diagnosing and classifying major retinal diseases. Sci Rep. 2022;12:19287. 6. Kumar V, Surve A, Kumawat D, et al. Ultra-wide field retinal imaging: a wider clinical perspective. Indian J Ophthalmol. 2021;69:824-35. 7. Salz JJ, Seibel BS. Screening with widefield fundus photography. Widefield technology has value for a practice’s patients, efficiency and bottom line. Ophthamology Management. June 1, 2019. Accessed August 16, 2023. 8. Purbick RM, Izadi S. Gupta A, Chong NV. Comparison of Optomap ultrawide-field imaging versus slit-lamp biomicroscopy for the assessment of diabetic retinopathy. Clin Ophthalmol. 2014;8:1413-7. 9. Wilson PJ, Ellis JD, MacEwen CJ, et al. Screening for diabetic retinopathy: a comparative trial of photograph and scanning laser ophthalmoscopy. Ophthalmologica. 2010;224:251-7. 10. Neubauer AS, Kernt M, Haritoglou C, et al. Nonmydriatic screening laser ophthalmoscopy (optomap). Graefes Arch Clin Exp Ophthalmol. 2008; 246:229-35. 11. Wessel MM, Aaker GD, Parlitis G, et al. Ultra-wide field angiography improves the detection and classification of diabetic retinopathy. Retina. 2012;32(4):785-91 12. Silva PS, Cavallerano JD, Sun JK, et al. Peripheral lesions identified by mydriatic ultrawide field imaging: distribution and potential impact on diabetic retinopathy severity. Ophthalmology. 2013;120(12):2587-95. 13. Silva PS, Cavallerano JD, Tolls D, et al. Potential efficiency benefits of nonmydriatic ultrawide field retinal imaging in an ocular telehealth diabetic retinopathy program. Diabetes Care. 2014;37(1):50-5. 14. Price LD, Au S, Chong NV. Optomap ultrawide field imaging identifies additional retinal abnormalities in patients with diabetic retinopathy. Clin Ophthalmol. 2015;9:527-31. 15. Aiello LP, Odia I, Glassman AR, et al. Comparison of early treatment diabetic retinopathy study standard 7-field imaging with ultrawide-field imaging for determining severity of diabetic retinopathy. JAMA Ophthalmol. 2019;137(1):65-73. 16. Silva PS, Cavallerano JD, Haddad NMN, et al. Peripheral lesions identified on ultrawide field imaging predict increased risk of diabetic retinopathy progression over four years. Ophthalmology. 2015;122:949-56. 17. Hirano T, Imai A, Kasamatsu H, et al. Assessment of diabetic retinopathy using two ultra-wide-field fundus imaging systems, the Clarus and Optos systems. BMC Ophthalmol. 2018;18(1):332. 18. Marcus DM, Silva PS, Liu D, et al. Association of predominantly peripheral lesions on ultra-widefield imaging and the risk of diabetic retinopathy worsening over time. JAMA Ophthalmol. 2022;140(10):946-54. 19. Silva PS, Liu D, Glassman AR, et al. Assessment of fluorescein angiography nonperfusion in eyes with diabetic retinopathy using ultrawide field retinal imaging. Retina. 2022;42:1302-10. 20. Yusuf IH, Lotery AJ. Thinking outside the circle-the potential value of ultra-widefield imaging. JAMA Ophthalmol 2022;140(10):955-6. 21. Nicholson L, Ramu J, Chan EW, et al. Retinal nonperfusion characteristics on ultra-wide field angiography in eyes with severe nonproliferative diabetic retinopathy and proliferative diabetic retinopathy. JAMA Ophthalmol. 2019;137:626-31. 22. Lasave AF, Shoughy SS, Kozak I, Arefalo, JF. When ultra-widefield retinal imaging is most useful. Retina Today. 2019;36-40. 23. Wykoff CC, Nittala MG, Zhou B et al. Intravitreal afilbercept for retinal nonperfusion in proliferative diabetic retinopathy: outcomes from the randomized recovery trial Ophthalmol Retina. 2019;3:1076-86. 24. Campochiaro PA. Low risk to retina from sustained suppression of VEGF. J Clin Invest. 2019;129(8):3029-31. 25. Pichi F, Carreño E, Pavesio C, et al. Consensus-based recommendations for OCT angiography reporting in uveitis. Br J Ophthalmol. 2023;107(7):959-65. 26. Munk MR, Kashani AH, Tadayoni R, et al. Recommendation for OCT angiography reporting in retinal vascular disease. A Delphi approach by international experts. Opthalmol Retina. 2022;6(9):753-61. 27. Li M, Mao M, Wei D, et al. Different scan areas affect the detection rates of diabetic retinopathy lesions by high-speed ultra-widefield swept-source optical coherence tomography angiography. Front Endocrinol (Lausanne). 2023;14:1111360. 28. Singer M, Tan CS, Bell D, Sadda SR. Area of peripheral retinal nonperfusion and treatment response in branch and central retinal vein occlusion. Retina. 2014;34(9):1736-42. 29. Campochiaro PA, Bhistkul RB, Shapiro H, Rubio RG. Vascular endothelial growth factor promotes progressive retinal nonperfusion in patients with retinal vein occlusion. Ophthalmology. 2013;120(4):795-802. 30. Tan TE, Ibrahim F, Chandrasekaran PR, Teo KYC. Clinical utility of ultra-widefield fluorescein angiography and OCT angiography for retinal vein occlusions. Front Med (Lausanne). 2023;10:1110166. 31. Sivaprasad S, Amoaku WM, Hykin P. The Royal College of Ophthalmologist Guidelines on retinal vein occlusions: executive summary. Eye (Lond). 2015;29(12):1633-8. 32. Nicholson L, Vazquez AC, Patrao NV, et al. Retinal nonperfusion in the posterior pole is associated with increased risk of neovascularization in central retinal vein occlusion. Am J Ophthalmol. 2017;182:118-25. 33. Nicholson L, Vazquez-Alfageme C, Sen P, et al. The clinical relevance of ultra-widefield angiography findings in patients with central retinal vein occlusion and macular oedema receiving anti-VEGF therapy. Eye (Lond). 2022;36(5):1086-93. 34. Witmer MT, Cho M, Favarone G, et al. Ultra-wide field autofluorescence imaging in non-traumatic rhegmatogenous retinal detachment. Eye (Lond). 2012;26(9):1209-16. 35. Choudhry N, Golding J, Manry MW, Rao RC. Ultra-widefield steering-based spectral-domain optical coherence tomography imaging of the retinal periphery. Ophthalmology. 2016;123:1368-74. 36. Alone A, Chandra K, Chhablani J. Wide-field imaging-an update. Indian J Ophthalmol. 2021;69(4):788-9. 37. Temkar S, Azad SV, Chawla R, et al. Ultra-widefield fundus fluorescein angiography in pediatric retinal vascular diseases. Indian J Ophthalmol. 2019;67(6):788-94. 38. Lee SJ, Yang KM, Lee KB, Park NC. Design of illumination system using characterized illuminances for smartphone-based fundus camera. Opt Lasers Eng. 2023;168:107664. 39. Iqbal, U. Smartphone fundus photography: a narrative review. Int J Retin Vitr. 2021;7(44). 40. Sedova A, Hajdu D, Datlinger G, et al. Comparison of early diabetic retinopathy staging in asymptomatic patients between autonomous AI-based screening and human-graded ultra-widefield color fundus images. Eye. 2022;36:510-6. |

