 |
A 49-year-old woman presented with a red, mildly painful left eye. She wore daily contact lenses, and her acuity was 20/20 OU. She manifested a small area of corneal infiltration, with overlying epithelial excavation and a mild anterior chamber reaction. The infiltrative lesion was well away from her visual axis. She was diagnosed clinically with a presumptive bacterial keratitis and empirically treated with moxifloxacin every two hours while awake. Over the next 10 days, she slowly healed, and the medication was discontinued.
Three weeks later, she came back with similar symptoms. At this time, her left eye had an area of infiltration and overlying excavation in a different area on her cornea. The original lesion had left a faint stromal scar; thus, this was a new infectious event. To aid in the diagnosis, polymerase chain reaction (PCR) testing, or molecular diagnostic testing, was performed. This test rapidly searches for microbial DNA and doesn’t involve culturing for growth.
Molecular diagnostics have changed the way we think about diagnosis of infectious disease. Aiding in diagnosis and therapeutic selection in bacterial keratitis management, it has made its way into clinical practice due to improved accessibility of commercially available test kits.
Infectious keratitis encompasses microbial causes (bacterial, fungal or parasitic) and viral keratitis.1 Of all microbial keratitis cases, 90% are bacterial in nature.1 While most cases of bacterial keratitis are diagnosed and successfully treated empirically with commercial broad-spectrum topical ophthalmic antibiotics, corneal culture is recommended for corneal infiltrates that are: (1) greater than 2mm, centrally located and/or associated with significant stromal involvement; (2) unresponsive to broad spectrum antibiotic treatment; (3) present in a patient with a history of corneal surgery; (4) atypical in clinical appearance; or (5) present with multiple infiltrates.2,3
Corneal culture and a corneal smear have long been considered the gold standard in identifying pathogenic organisms in bacterial keratitis and form the basis for antimicrobial sensitivity testing. However, its associated practical challenges mean that access is primarily limited to academic subspecialty settings.4
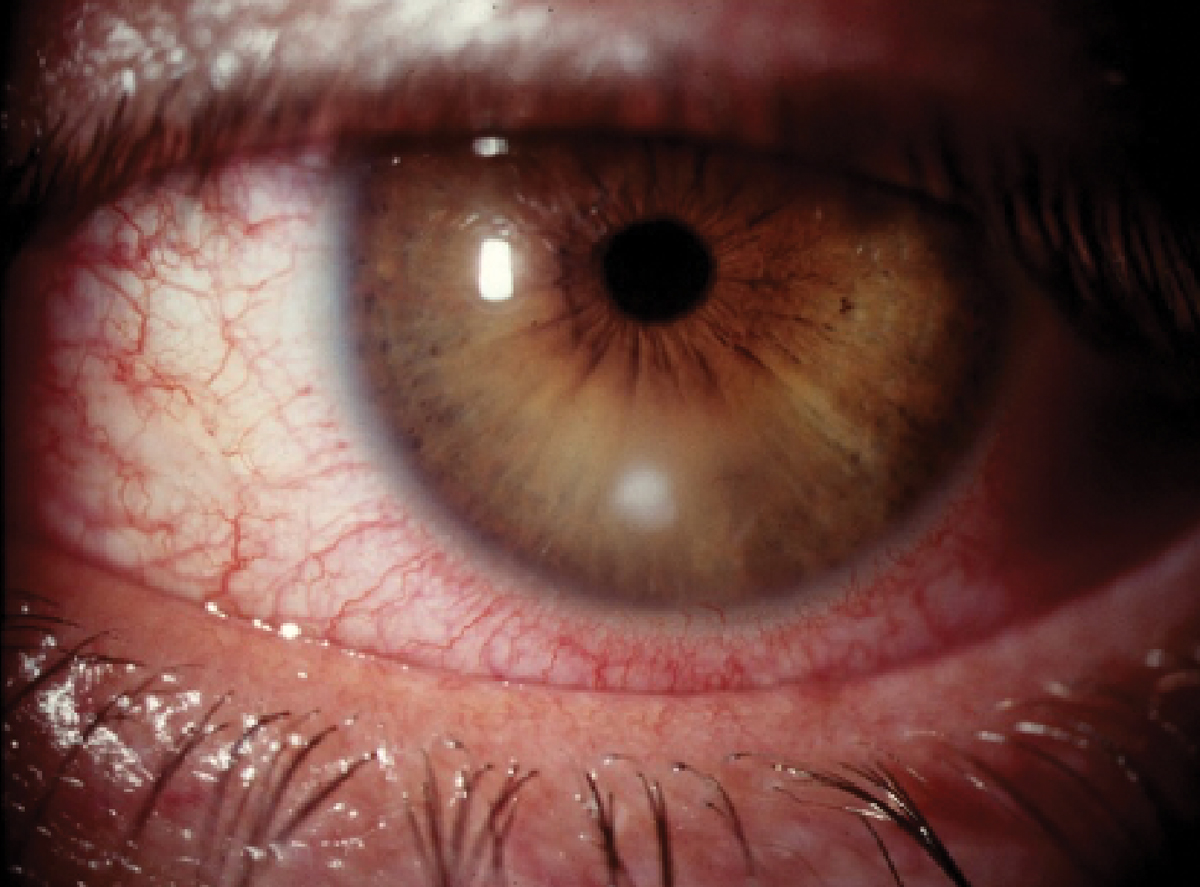 |
|
Bacterial keratitis in another patient. Molecular diagnostic testing has illuminated that most of these clinically suspicious lesions are in fact infectious. Click image to enlarge. |
The Basics
PCR technology used in molecular diagnostic testing requires a DNA template that contains the known target sequence, the sample DNA, heat-stable DNA polymerase, which synthesizes complementary DNA to the target sequence, and a primer sequence made of synthetic DNA fragments that bounds the sequence of interest.5 During repetitive cycling of temperature in the system, the primer binds to a specific sequence in the sample each cycle, DNA is amplified and the newly produced strand of DNA is dissociated from the primer sequence. PCR allows for up to billions of copies of DNA to be produced from a single molecule of DNA rapidly.5 Detection of bacterial 16S ribosomal DNA and fungal 18S ribosomal DNA in ocular samples are excellent broad-range targets for pathogen detection due to their commonality across bacterial and fungal species, respectively.5,6
The Ins and Outs
Only approximately half of corneal cultures in clinically diagnosed infectious keratitis cases detect potential pathogenic growth.7 While false negatives occur in molecular testing as well, there may be improved detection of bacterial presence. Also, unexpected or atypical organisms may be more likely to be detected through molecular testing due to difficulties of certain organisms of in vitro growth.3,4,7
More than one causative organism may be responsible for pathogenesis in infectious keratitis. In an analysis of positive cultures among infectious keratitis patients, 43% of positive cultures yielded at least two bacterial species, and while broad-spectrum antibiotics may be effective against many common corneal pathogens, identifying all causative bacteria and determining individual susceptibility patterns can best inform and individualize treatment.2,8 Isolating bacteria of different genera and species by culture from a single sample requires plating on specific agar media suited to the bacteria’s nutritional and environmental needs, which is a cumbersome task.2 Molecular testing of biologic samples can identify a variety of causative organisms from a single sample, without plating or incubation requirements, increasing the likelihood of detection of all present organisms.
The potential downside to detecting and amplifying trace amounts of microbial DNA is that naturally occurring, non-pathogenic conjunctival and eyelid flora found in low numbers may be inadvertently included in the sample along with the pathogenic organism of the infectious condition, leading to potential false positives.4,9,10 In a sample where multiple organisms have been identified, determining which organisms are most likely to represent the pathogenic bacteria presents yet another clinical diagnostic challenge.4,9,10 In a molecular diagnostic report where multiple microbes have been detected, those with the greatest number of detectable copies are more likely to be the cause of the underlying pathology.
While sensitivity and specificity of molecular testing and corneal culture cannot be described or directly compared due to lack of an error-free reference standard, it is possible to compare pathogen detection from the same sample by each method.3 Of 272 eyes of individuals with clinically diagnosed infectious keratitis in a study, the diagnostic efficacy measured by area under the curve (AUC) was similar between culture and detection of bacterial DNA at 0.65 and 0.67, respectively.6 The ability to detect a bacterial pathogen was improved when both were performed on a sample, with the AUC increasing to 0.72, demonstrating the adjunctive effect improving diagnostic accuracy.6
Despite the practical challenges associated with corneal culture and the convenience of commercially available molecular test kits for ophthalmic indications, molecular testing should not be considered a replacement for corneal culture when indicated by current clinical practice guidelines but as adjunctive analysis instead.2,3,11 With a laboratory turnaround time of approximately 24 hours, the results of molecular testing, including pathogen detection and susceptibility findings, should be used to alter therapy if needed or to determine if current therapy is sufficient.3
In the case presented, two days after corneal scraping for molecular testing, the patient’s results were returned. Her lesion revealed three isolates: the anaerobic bacteria Pepostreptococcus anaeorbius (moderate load at 320,000 copies/mL) and Cutibacterium acnes (moderate load at 170,000 copies/mL), as well as coagulase-negative Staphylococcus epidermidis (moderate load at 600,000 copies/mL). Included susceptibility testing identified Pepostreptococcus and Staphylococcus, which were both readily susceptible to the prescribed topical moxifloxacin, but Cutibacterium was only variably sensitive to that agent.
According to the report, Cutibacterium was very sensitive to oral doxycycline, and this was added at 100mg twice daily by mouth to her topical regimen. With this addition to topical ophthalmic moxifloxacin, her infiltrate resolved rapidly and completely. The organism resistant to moxifloxacin was likely the reason the patient previously healed comparatively slowly and rebounded shortly after initial treatment.
Takeaways
There are several lessons learned from molecular diagnostic testing evaluation. First, the majority of these suspicious infiltrates that are typically diagnosed and effectively managed empirically may actually represent true microbial infection. Second, there are often multiple microbes responsible for pathogenesis, with differing resistance profiles to commonly-used, broad-spectrum topical antibiotics. Finally, when treating presumed bacterial keratitis empirically, choosing more than one topical ophthalmic agent, each with variation in known susceptibility patterns for common ocular isolates, can increase the likelihood of optimal coverage in cases of polymicrobial infection.
As we shift toward true personalized care, the incorporation of diagnostic testing platforms that are accessible, precise and affordable in an acute care community environment can aid our diagnostic abilities and provide the basis for formulating a truly individualized treatment plan for individuals with corneal infectious disease.
Dr. Steen is an assistant professor at Nova Southeastern University College of Optometry where she serves as director of the Glaucoma Service, coordinator of the Primary Care with Emphasis in Ocular Disease Residency and teaches courses in glaucoma and ocular pharmacology. Her financial disclosures include Bausch & Lomb, Santen, Ocuphire and Carl Zeiss Meditec.
Dr. Sowka is an attending optometric physician at Center for Sight in Sarasota, FL, where he focuses on glaucoma management and neuro-ophthalmic disease. He is a consultant and advisory board member for Carl Zeiss Meditec and Bausch Health.
1. Durand ML, Barshak MB, Chodosh J. Infectious keratitis in 2021. JAMA. 2021;326(13):1319-20. 2. Lin A, Rhee MK, Akpek EK, et al. American Academy of Ophthalmology Preferred Practice Pattern Cornea and External Disease Panel. Bacterial Keratitis Preferred Practice Pattern®. Ophthalmology. 2019;126:1-55. 3. Tuft S, Bunce C, De S, et al. Utility of investigation for suspected microbial keratitis: a diagnostic accuracy study. Eye. 2023;37(3):415-20. 4. Ting D, Chodosh J, Mehta J. Achieving diagnostic excellence for infectious keratitis: a future roadmap. Front Microbiol. 2022;13:1020198. 5. Van Gelder R. Molecular diagnostics for ocular infectious diseases: LXXVIII Edward Jackson Memorial Lecture. Am J Ophthalmol. 2022;235:300-12. 6. Shimizu D, Miyazaki D, Ehara F, et al. Effectiveness of 16S ribosomal DNA real-time PCR and sequencing for diagnosing bacterial keratitis. Graefes Arch Clin Exp Ophthalmol. 2020;258(1):157-66. 7. Ung L, Bispo PJM, Shanbhag SS, et al. The persistent dilemma of microbial keratitis: Global burden, diagnosis, and antimicrobial resistance. Surv Ophthalmol. 2019;64(3):255-71. 8. Pakzad-Vaezi K, Levasseur SD, Schendel S, et al. The corneal ulcer one-touch study: a simplified microbiological specimen collection method. Am J Ophthalmol. 2015;159(1):37-43. 9. Kim E, Chidambaram JD, Srinivasan M, et al. Prospective comparison of microbial culture and polymerase chain reaction in the diagnosis of corneal ulcer. Am J Ophthalmol. 2008;146(5):714-23. 10. Salter SJ, Cox MJ, Turek EM, et al. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 2014;12:87. 11. An N, Wang C, Dou X, et al. Comparison of 16S rDNA amplicon sequencing with the culture method for diagnosing causative pathogens in bacterial corneal infections. Trans. Vis Sci Technol. 2022;11(2):29. |

