
As most readers probably know, the landmark Age-Related Eye Disease Study (AREDS) demonstrated that a nutritional supplement could positively influence progression of AMD.1
Since that 2001 study appeared, a big question has been: might nutritional supplements also have a similar effect on diabetic retinopathy (DR)? Because vitamins, minerals and other micronutrients serve a variety of biological functions potentially beneficial in diabetes, there has been renewed interest in the possibility of these supplements treating a host of diabetes complications.
Long-term administration of AREDS antioxidants has yielded exciting results in preventing the pathogenesis of DR in rodent models.
“These results suggest the merit of testing the AREDS antioxidants in a clinical trial to prevent the development and/or progression of diabetic retinopathy, with the possibility of reducing the impact of this common vision-threatening disease,” wrote prominent retinal biologists in a 2011 Investigative Ophthalmology & Visual Science paper.2
We can safely say there is a plausible epidemiological basis for thinking that dietary supplementation might correct nutritional deficiencies common in diabetes. Studies suggest that patients with diabetes are more likely to suffer from deficiencies of potassium, calcium, magnesium, zinc, manganese, chromium, vitamin D and serum carotenoids.3-8
Such deficiencies may negatively affect glucose control, cell repair and survival, and may ultimately contribute to diabetes complications. Moreover, long-term use of the most widely prescribed oral anti-diabetic agent, metformin, has recently been linked with vitamin B12 deficiency, a risk factor for diabetic retinopathy.9,10
But will merely correcting micronutrient deficiencies make a meaningful difference in terms of the development and progression of DR in humans? Another, related question: might additional preventative micronutrients be helpful?
Of course, only a large, well-designed clinical trial would give us the answer. In the meantime, however, we combine our knowledge of the complex biological pathways that lead to diabetic retinopathy with evidence that shows how targeted micronutrients block these pathways to make scientifically rational recommendations to our patients with diabetes.
Current clinical algorithms for diabetic eye disease call for earlier diagnosis of diabetes, tighter metabolic control, annual dilated retinal examinations and treatment (e.g., laser, anti-VEGF drugs, steroids) if DR threatens vision. There is no doubt this “reactive” strategy has protected the vision of millions of diabetes patients, but we also know there is room for improvement. For example, despite the enormous effort put into education, many patients with diabetes do not undergo dilated exams or achieve their metabolic targets—and, furthermore, even those who strictly follow these guidelines may still develop sight-threatening diabetic retinopathy (STR).
A major strength of the targeted nutritional supplements discussed in this article is their ability to offer a proactive, rather than reactive, strategy in fighting this disease—an ounce of prevention that might ultimately be worth the proverbial pound of cure.
A Continuous Climb
Diabetic retinopathy remains the leading cause of new cases of blindness in Americans under age 74. This may be surprising to many practitioners who have noticed a long-term trend towards reduced rates of severe DR over the last several decades. Despite evidence that tighter control of blood glucose and blood pressure reduces the risk of microvascular diabetes complications, as well as tremendous advances in the clinical management of diabetic eye disease, rates of DR in the US have increased by 89% over the last decade (probably due to better detection and increasing rates of diabetes).11
Importantly, significant visual impairment associated with diabetes remains high, and recent estimates show that nearly 5% of US adults with diabetes have STR––with significantly higher rates amongst African, Latino and Native American populationss.12 Moreover, global estimates of DR and STR based on pooled analysis of population-based studies show 93 million cases of DR and 28 million cases of STR, which suggests that nearly 2.8 million Americans may have sight-threatening diabetic retinopathy today.13
Increasing the rates of dilated eye exams among patients with diabetes reduces blindness, yet nearly 40% of patients failed to have one performed in 2010.14 We also know that improving blood glucose and blood pressure control significantly reduces rates of DR and STR. Nonetheless, 12% of diabetes patients had HbA1c levels >9% in 2004, while more than 30% had HbA1c levels >8%.15 Although improving blood glucose control lowers risk, evidence shows that there is no level of average blood glucose that fully protects against DR.
Perhaps the single most important step we can take to combat DR is to reduce the incidence of diabetes (especially type 2) by addressing complex environmental factors like obesity, sedentary lifestyle and even sleep deprivation.16,17 Rates of diabetes continue to climb, and projections are that one in three American adults will have it by 2050.18
Working Model of DR
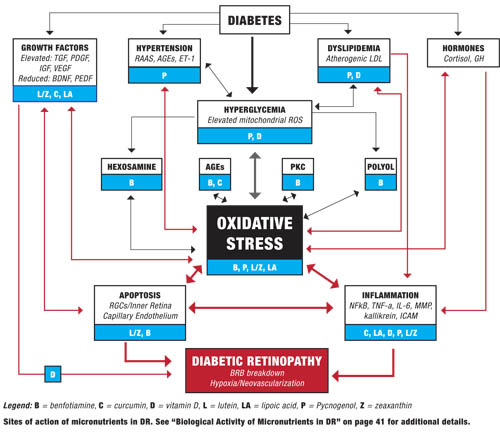
To better understand how and why nutritional supplements might benefit diabetic retinopathy, it is helpful to have a working model of the biology underlying the disease.
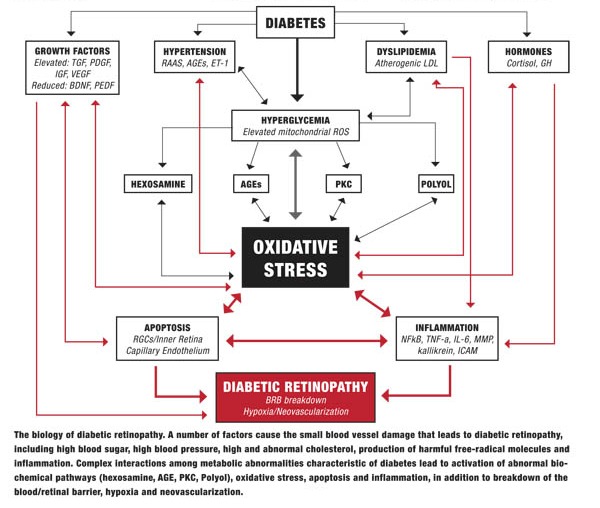
Diabetes causes a number of metabolic abnormalities, including hyperglycemia, dyslipidemia, hypertension and oxidative stress. These factors interact in a variety of ways to cause increased production of singlet oxygen (also known as reactive oxygen species [ROS]) by mitochondria, especially within the retina and other insulin-independent tissues (small-caliber neurons, renal glomeruli and aorta). These mitochondrial ROS activate specific and well-established biochemical pathways within retinal cells that directly lead to vascular and retinal ganglion cell damage (polyol, PKC, hexosamine flux and advanced glycation endproduct pathways).19,20
Release of inflammatory proteins and programmed destruction (apoptosis) of capillary endothelial cells and retinal ganglion cells may lead to breakdown of the blood/retinal barrier, resulting in vascular leakage, hypoxia and retinal neovascularization—the hallmarks of diabetic retinopathy. Accompanying deficits in visual function, including contrast sensitivity, visual field sensitivity and color vision, are common.
Elevated blood glucose plays a central role in DR, worsening dyslipidemia and hypertension, and driving oxidative stress that damages the retina. This is why control of blood sugar (as well as blood pressure and blood lipids) constitutes the foundation of preventing diabetes complications, including retinopathy. However, a number of biological processes that occur further downstream from hyperglycemia also can affect progression and thus give us additional targets for intervention.
The Benefits of Nutritional Supplementation
When considering evidence for micronutrient intervention in DR pathobiology, bear in mind that effects demonstrated in a laboratory, or associations seen in human beings, do not necessarily translate into
 |
|
 |
|
 |
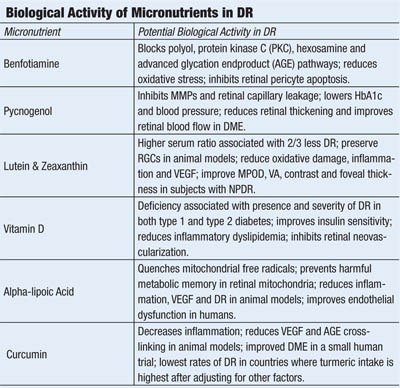
• Benfotiamine. A synthetic, lipophilic analog of vitamin B1 (thiamine), benfotiamine has been shown to elevate intracellular levels of transketolase––an enzyme that redirects glucose and the harmful glucose metabolites, fructose-6-phosphate and glyceraldehyde-3-phosphate, to the pentose phosphate shunt pathway. Increased transketolase levels reduce intracellular formation of protein kinase C, sorbitol, advanced glycation (AGE) and advanced lipo-oxidation (ALE) endproducts—substrates directly involved in the pathogenesis of diabetic microvascular damage, including diabetic retinopathy.21
Benfotiamine has been shown to block activity in the polyol, hexosamine flux, protein kinase C and advanced glycation endproduct pathways, helping to prevent diabetic retinopathy in a murine model of the disease.22 It also prevents glucose-dependent apoptosis of human retinal pericytes.23
Multiple studies have shown that benfotiamine improves vibratory sensation, nerve conduction and pain scores in patients with diabetic neuropathy.24,25 When combined with slow-release alpha-lipoic acid, benfotiamine normalized activity in the polyol, hexosamine and AGE pathways in patients with type 1 diabetes, as well as reduced levels of prostacyclin synthase––a protein implicated in atherosclerosis.26 Benfotiamine also prevented endothelial dysfunction and oxidative stress in patients with type 2 diabetes following a provocative high-AGE meal.27
• Pycnogenol. A patented extract of bark from the French Maritime Pine, Pinus pinaster, pycnogenol contains a number of polyphenolic procyanidins (anthocyanidins)––antioxidants that inhibit inflammation and selectively bind to collagen and elastin fibers to improve capillary fragility.28,29 Pycnogenol inhibits nuclear factor kappa beta (NF-kβ) and secretion of MMP-1, MMP-2 and MMP-9, of which the latter two have been implicated in breakdown of the blood/retinal barrier in diabetic retinopathy.30, 31
Pycnogenol also inhibits the digestive enzyme alpha-glucosidase, delaying the intestinal absorption of sugars to ameliorate post-prandial hyperglycemia.32 More than 30 randomized, controlled clinical trials have shown that pycnogenol improves a number of health conditions, ranging from tinnitus to chronic venous insufficiency to kidney dysfunction.33-35 In patients with type 2 diabetes, Pycnogenol improved glycosylated hemoglobin, blood pressure and dyslipidemia.36,37
Several studies suggest that Pycnogenol improves capillary leakage and retards retinopathy progression in patients with diabetes.38,39 Pycnogenol was shown to improve retinal blood flow, reduce retinal thickening and improve visual acuity in patients with early diabetic macular edema.40
• Non-provitamin A carotenoids. Lutein and zeaxanthin are the primary components of the macular pigment, which filters short-wavelength blue light, scavenges free radicals and suppresses inflammation in a number of disease models, including streptozotocin-induced diabetes and retinal ischemia.41
Type 2 diabetes patients with a higher ratio of serum non-pro-vitamin A carotenoids (lutein, zeaxanthin, lycopene) to pro-vitamin A carotenoids (alpha-carotene, beta-carotene and beta-cryptoxanthin) were associated with a 66% reduction in risk for diabetic retinopathy after adjustment for confounding variables.42 Moreover, macular pigment optical density (MPOD) has been reported to be lower in patients with type 2 diabetes than in age-matched controls, lower still in patients with type 2 diabetes and retinopathy, and inversely correlated with glycosylated hemoglobin.43
Lutein supplementation reduced retinal oxidative stress and activation of NF-kβ, and increased the neuroprotective cytokine brain-derived neurotrophic factor (BDNF) in a mouse model.44 Diabetic mice supplemented with lutein/zeaxanthin-rich wolfberry demonstrated normalized retinal thickness, RPE integrity and number of retinal ganglion cells via initiation of adenosine monophosphate-activated protein kinase and reduction of endoplasmic reticulum stress compared with controls.45
Supplementation with zeaxanthin also prevented oxidative damage and reduced production of VEGF and intracellular adhesion molecule (ICAM-1) in diabetic rats.46 Lutein (6mg per day) and zeaxanthin (0.5mg per day) supplementation over three months increased MPOD, improved visual acuity, contrast sensitivity and foveal thickness in subjects with non-proliferative diabetic retinopathy compared to controls.47
• Vitamin D. Low serum levels of vitamin D have been implicated in the development of both type 1 and type 2 diabetes.48-50 Vitamin D insufficiency also is a risk factor for atherosclerosis and cancer––both of which are more common in patients with diabetes.51,52 Recently, serum vitamin D status has been linked to the presence and severity of diabetic retinopathy in patients with both type 1 and type 2 diabetes; vitamin D deficiency also proved more predictive of DR than diabetes duration or glycosylated hemoglobin in Australian adolescents with type 1 diabetes.53,54
Vitamin D supplementation reduced both vascular endothelial growth factor (VEGF) and transforming growth factor beta-1 (TGF-β1) in a murine model of diabetic retinopathy, giving insight into its possible mechanism of action affecting DR.55 In a murine model of oxygen-induced ischemic retinopathy, supplementation with the active metabolite of vitamin D3 (1,25-dihydroxyvitamin D3) significantly inhibited retinal neovascularization without reducing ocular VEGF levels.56 Vitamin D also appears to block foam cell formation in serum samples from subjects with type 2 diabetes, suggesting improvement of the inflammatory dyslipidemia that contributes to DR.57,58
• Alpha-lipoic acid. Also known as thioctic acid, alpha-lipoic acid (ALA) is a naturally occurring compound synthesized by mitochondria that serves as a cofactor for enzymes producing ATP via the citric acid cycle.59 In addition to being a potent antioxidant, ALA stimulates synthesis of glutathione and regenerates other antioxidants by reducing their oxidized forms—including vitamin C, vitamin E and coenzyme Q10—an attribute that has given ALA a reputation as the “antioxidant of antioxidants.”
Because alpha-lipoic acid crosses the blood/brain barrier and distributes to mitochondria where its reduced form, dihyrolipoic acid (DHLA), is capable of regenerating other antioxidants, it may be particularly well-suited to pathologies characterized by excess mitochondrial production of reactive oxygen species, such as diabetes.19
ALA has been shown to reduce both small and large blood vessel complications of diabetes in animal models.60,61 ALA treatment reduced retinal capillary damage, oxidative stress, nuclear factor kappa-beta activation and VEGF in an animal model of diabetic retinopathy. 62 Also, long-term use prevented the development of retinopathy in diabetic rats.63
Human RPE cells are protected from hyperglycemic oxidative stress and mitochondrial dysfunction when treated with ALA.64 Recent evidence suggests ALA prevents deleterious “metabolic memory” (i.e., worsening of diabetic retinopathy after an initial period of poor blood glucose control, despite subsequent improved blood glucose control) by improving mitochondrial homeostasis.65 ALA supplementation improved “endothelial dysfunction” (flow-mediated vasodilation) in patients with metabolic syndrome and impaired fasting glucose.66 Though administration to human subjects with type 2 diabetes over two years did not result in statistically significant reductions in clinically significant macular edema, subjects in the treatment arm had a 14% lower relative risk of developing it.67
• Curcumin. This is a component of the Indian spice turmeric. It contains a number of polyphenolic molecules believed to modulate inflammation. Curcumin inhibited diabetes-induced elevation in levels of the retinopathic proteins IL-1β, VEGF and NF-kβ in a murine model of diabetes, without inducing amelioration of hyperglycemia, and prevents AGE-collagen cross linking in diabetic rats.68,69 Curcumin also was shown to normalize elevated VEGF and tumor necrosis factor-alpha (TNF-α), the inflammatory cytokine, as well as to prevent early basement membrane changes associated with diabetic retinopathy in an animal model.70
Additionally, lecithinized curcumin was shown to improve retinal blood flow, retinal edema and visual acuity in subjects with DR over four weeks of supplementation.71 It is noteworthy that DR rates are lower in Southern India––where turmeric consumption is highest––than in European and North American populations, after controlling for established risk factors.72
Yes, But Does Dietary Supplementation Work?
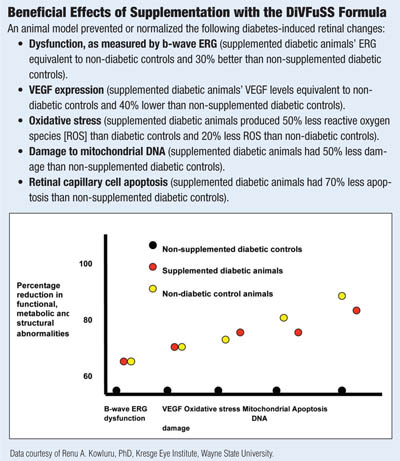
I have seen beneficial effects with some of these micronutrients in my practice. This, of course, proves nothing. Only a large clinical trial will tell, but I am currently undertaking a small trial in my office using a combination of these supplements in patients with diabetes and mild to moderate non-proliferative diabetic retinopathy. The study aims to determine effects on visual function, spectral-domain optical coherence tomography results, and serum markers of inflammation (the Diabetes Visual Function Supplement Study – DiVFuSS; ClinicalTrials.gov Identifier: NCT01646047; Sponsored by ZeaVision, LLC).
In a 2013 ARVO poster, the test formula was shown to improve or normalize retinal structure, metabolism and function in an animal model of DR.73 Specifically, it reduced mitochondrial damage, oxidative stress, activation of NF-kβ—as well as production of VEGF, ICAM-1 and MMP. Retinal function as assessed by β-wave ERG was preserved and, most significantly, apoptosis of retinal capillary cells was prevented without causing significant difference in blood glucose levels in treated and untreated animals.
Diabetic retinopathy continues to be a leading cause of vision loss, despite improvements in metabolic control and advancements in treatment. Though annual dilated eye exams make a tremendous difference in detecting and treating sight-threatening disease, many patients are still falling through the cracks. Though tighter blood sugar and blood pressure control certainly lowers patient risk, many do not achieve these goals, and some patients develop severe DR despite excellent control.
Use of targeted micronutrients may give us the opportunity to offer patients with diabetes something more than warnings about good disease control and regular dilated eye exams. It may give us a chance to do more than simply monitor for vision threatening diabetic retinopathy and eventual referral to retinal specialists for treatment. It offers an opportunity to be proactive, rather than merely reactive, by addressing some of the biological mechanisms underlying the disease.
Dr. Chous specializes in diabetes eye care and education in Tacoma, Wash. He is a consultant to several diabetes-related companies and is receiving support for DiVFuSS from ZeaVision, LLC.
1. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001 Oct;119(10):1417-36.
2. Kowluru RA, Zhong Q. Beyond AREDS: is there a place for antioxidant therapy in the prevention/treatment of eye disease? Invest Ophthalmol Vis Sci. 2011 Nov 7;52(12):8665-71.
3. Afridi HI, Kazi TG, Kazi N, et al. Potassium, calcium, magnesium, and sodium levels in biological samples of hypertensive and nonhypertensive diabetes mellitus patients. Biol Trace Elem Res. 2008 Sep;124(3):206-24.
4. Simmons D, Joshi S, Shaw J. Hypomagnesaemia is associated with diabetes: Not pre-diabetes, obesity or the metabolic syndrome. Diabetes Res Clin Pract. 2010 Feb;87(2):261-6.
5. Kazi TG, Afridi HI, Kazi N, et al. Copper, chromium, manganese, iron, nickel, and zinc levels in biological samples of diabetes mellitus patients. Biol Trace Elem Res. 2008 Apr;122(1):1-18
6. Parker J, Hashmi O, Dutton D, et al. Levels of vitamin D and cardiometabolic disorders: systematic review and meta-analysis. Maturitas. 2010 Mar;65(3):225-36.
7. Takiishi T, Gysemans C, Bouillon R, Mathieu C. Vitamin D and diabetes. Endocrinol Metab Clin North Am. 2010 Jun;39(2):419-46.
8. Coyne T, Ibiebele TI, Baade PD, et al. Metabolic syndrome and serum carotenoids: findings of a cross-sectional study in Queensland, Australia. Br J Nutr. 2009 Dec;102(11):1668-77.
9. de Jager J, Kooy A, Lehert P, et al. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial. BMJ. 2010 May 20;340:c2181.
10. Satyanarayana A, Balakrishna N, Pitla S, et al. Status of B-vitamins and homocysteine in diabetic retinopathy: association with vitamin-B12 deficiency and hyperhomocysteinemia. PLoS One. 2011;6(11):e26747.
11. National Eye Institute Statement, November 2012. Sharp rise in diabetic eye disease makes American Diabetes Month ever more important. Available at: www.nei.nih.gov/news/statements/diabetesmonth2012.asp. Accessed August 6, 2013.
12. Zhang X, Saaddine JB, Chou CF, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010 Aug 11;304(6):649-56.
13. Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012 Mar;35(3):556-64.
14. Centers for Disease Control. Age-Adjusted Percentage of Adults Aged 18 Years or Older with Diagnosed Diabetes Receiving a Dilated Eye Exam in the Last Year, United States, 1994–2010. Available at: www.cdc.gov/diabetes/statistics/preventive/fX_eye.htm. Accessed August 8, 2013.
15. Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care. 2008 Jan;31(1):81-6.
16. Crandall JP, Knowler WC, Kahn SE, et al. The prevention of type 2 diabetes. Nat Clin Pract Endocrinol Metab. 2008 Jul;4(7):382-93.
17. Lucassen EA, Rother KI, Cizza G. Interacting epidemics? Sleep curtailment, insulin resistance, and obesity.1 in 3 adults could have diabetes by 2050. Manag Care. 2010 Nov;19(11):47.
18. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005 Jun;54(6):1615-25.
19. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010 Oct 29;107(9):1058-70.
21. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005 Jun;54(6):1615-25.
22. Hammes HP, Du X, Edelstein D, et al. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003 Mar;9(3):294-9.
23. Beltramo E, Nizheradze K, Berrone E, et al. Thiamine and benfotiamine prevent apoptosis induced by high glucose-conditioned extracellular matrix in human retinal pericytes.
Diabetes Metab Res Rev. 2009 Oct;25(7):647-56.
24. Stracke H, Gaus W, Achenbach U, et al. Benfotiamine in diabetic polyneuropathy (BENDIP): results of a randomised, double blind, placebo-controlled clinical study. Exp Clin Endocrinol Diabetes. 2008 Nov;116(10):600-5.
25. Stracke H, Lindemann A, Federlin K A benfotiamine-vitamin B combination in treatment of diabetic polyneuropathy. Exp Clin Endocrinol Diabetes. 1996;104(4):311-6.
26. Du X, Edelstein D, Brownlee M. Oral benfotiamine plus alpha-lipoic acid normalises complication-causing pathways in type 1 diabetes. Diabetologia. 2008 Oct;51(10):1930-2.
27. Stirban A, Negrean M, Stratmann B, et al. Benfotiamine prevents macro- and microvascular endothelial dysfunction and oxidative stress following a meal rich in advanced glycation end products in individuals with type 2 diabetes. Diabetes Care. 2006 Sep;29(9):2064-71.
28. Tixier JM, Godeau G, Robert AM, Hornebeck W. Evidence by in vivo and in vitro studies that binding of pycnogenols to elastin affects its rate of degradation by elastases. Biochem Pharmacol. 1984 Dec 15;33(24):3933-9.
29. Grimm T, Schäfer A, Högger P. Antioxidant activity and inhibition of matrix metalloproteinases by metabolites of maritime pine bark extract (pycnogenol). Free Radic Biol Med. 2004 Mar 15;36(6):811-22.
30. Grimm T, Chovanová Z, Muchová J, et al. Inhibition of NF-kappaB activation and MMP-9 secretion by plasma of human volunteers after ingestion of maritime pine bark extract (Pycnogenol). J Inflamm (Lond). 2006 Jan 27;3:1.
31. Kowluru RA, Zhong Q, Santos JM. Matrix metalloproteinases in diabetic retinopathy: potential role of MMP-9. Expert Opin Investig Drugs. 2012 Jun;21(6):797-805.
32. Schäfer A, Högger P. Oligomeric procyanidins of French maritime pine bark extract (Pycnogenol) effectively inhibit alpha-glucosidase. Diabetes Res Clin Pract. 2007 Jul;77(1):41-6.
33. Grossi MG, Belcaro G, Cesarone MR, et al. Improvement in cochlear flow with Pycnogenol in patients with tinnitus: a pilot evaluation. Panminerva Med. 2010 Jun;52(2 Suppl 1):63-7.
34. Cesarone MR, Belcaro G, Rohdewald P, et al. Improvement of signs and symptoms of chronic venous insufficiency and microangiopathy with Pycnogenol: a prospective, controlled study. J Cardiovasc Pharmacol Ther 2010 Sep;17(11):835-9.
35. Cesarone MR, Belcaro G, Stuard S, et al. Kidney flow and function in hypertension: protective effects of pycnogenol in hypertensive participants--a controlled study. J Cardiovasc Pharmacol Ther. 2010 Mar;15(1):41-6.
36. Zibadi S, Rohdewald PJ, Park D, Watson RR. Reduction of cardiovascular risk factors in subjects with type 2 diabetes by Pycnogenol supplementation. Nutr Res. 2008 May;28(5):315-20.
37. Liu X, Wei J, Tan F, et al. Antidiabetic effect of Pycnogenol French maritime pine bark extract in patients with diabetes type II. Life Sci. 2004 Oct 8;75(21):2505-13.
38. Schönlau F, Rohdewald P. Pycnogenol for diabetic retinopathy. A review. Int Ophthalmol. 2001;24(3):161-71.
39. Spadea L, Balestrazzi E. Treatment of vascular retinopathies with Pycnogenol. Phytother Res. 2001 May;15(3):219-23.
40. Steigerwalt R, Belcaro G, Cesarone MR, et al. Pycnogenol improves microcirculation, retinal edema, and visual acuity in early diabetic retinopathy. J Ocul Pharmacol Ther. 2009 Dec;25(6):537-40.
41. Kijlstra A, Tian Y, Kelly ER, Berendschot TT. Lutein: more than just a filter for blue light. Prog Retin Eye Res. 2012 Jul;31(4):303-15.
42. Brazionis L, Rowley K, Itsiopoulos C. Plasma carotenoids and diabetic retinopathy. Br J Nutr. 2009 Jan;101(2):270-7.
43. Lima VC, Rosen RB, Maia M, et al. Macular pigment optical density measured by dual-wavelength autofluorescence imaging in diabetic and nondiabetic patients: a comparative study. Invest Ophthalmol Vis Sci. 2010 Nov;51(11):5840-5.
44. Sasaki M, Ozawa Y, Kurihara T, et al. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia. 2010 May;53(5):971-9.
45. Tang L, Zhang Y, Jiang Y,et al. Dietary wolfberry ameliorates retinal structure abnormalities in db/db mice at the early stage of diabetes. Exp Biol Med (Maywood). 2011 Sep 1;236(9):1051-63.
46. Kowluru RA, Menon B, Gierhart DL. Beneficial effect of zeaxanthin on retinal metabolic abnormalities in diabetic rats. Invest Ophthalmol Vis Sci. 2008 Apr;49(4):1645-51
47. Hu BJ, Hu YN, Lin S, et al. Application of Lutein and Zeaxanthin in nonproliferative diabetic retinopathy. Int J Ophthalmol. 2011 Mar;4(3):303-6.
48. Chakhtoura M, Azar ST. The role of vitamin d deficiency in the incidence, progression, and complications of type 1 diabetes mellitus. Int J Endocrinol. 2013;2013:148673.
49. Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr. 2011 Sep;65(9):1005-15.
50. Gorham ED, Garland CF, Burgi AA, et al. Lower prediagnostic serum 25-hydroxyvitamin D concentration is associated with higher risk of insulin-requiring diabetes: a nested case-control study. Diabetologia. 2012 Dec;55(12):3224-7.
51. Lee JI, Oh SJ, Ha WC, et al. Serum 25-hydroxyvitamin D concentration and arterial stiffness among type 2 diabetes.Diabetes Res Clin Pract. 2012 Jan;95(1):42-7.
52. Eaton CB, Young A, Allison MA, et al. Prospective association of vitamin D concentrations with mortality in postmenopausal women: results from the Women’s Health Initiative (WHI). Am J Clin Nutr. 2011 Oct 26. [Epub ahead of print]
53. Payne JF, Ray R, Watson DG, et al. Vitamin D deficiency in diabetic retinopathy. Endocr Pract. 2012 Mar-Apr;18(2):185-93.
54. Kaur H, Donaghue KC, Chan AK, et al. Vitamin D deficiency is associated with retinopathy in children and adolescents with type 1 diabetes. Diabetes Care. 2011 Jun;34(6):1400-2.
55. Ren Z, Li W, Zhao Q, et al. The impact of 1,25-dihydroxy vitamin D3 on the expressions of vascular endothelial growth factor and transforming growth factor-β₁ in the retinas of rats with diabetes. Diabetes Res Clin Pract. 2012 Oct 19. [Epub ahead of print].
56. Albert DM, Scheef EA, Wang S, et al. Calcitriol is a potent inhibitor of retinal neovascularization. Invest Ophthalmol Vis Sci. 2007 May;48(5):2327-34.
57. Oh J, Weng S, Felton SK, Bhandare S, et al. 1,25(OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009 Aug 25;120(8):687-98.
58. Busik JV, Esselman WJ, Reid GE. Examining the role of lipid mediators in diabetic retinopathy. Clin Lipidol. 2012 Dec 1;7(6):661-75.
59. Bilska A, Włodek L. Lipoic acid – the drug of the future? Pharmacol Rep. 2005 Sep-Oct;57(5):570-7.
60. Lin J, Bierhaus A, Bugert P, et al. Effect of R-(+)-alpha-lipoic acid on experimental diabetic retinopathy. Diabetologia. 2006 May;49(5):1089-96.
61. Yi X, Maeda N. alpha-Lipoic acid prevents the increase in atherosclerosis induced by diabetes in apolipoprotein E-deficient mice fed high-fat/low-cholesterol diet. Diabetes. 2006; 55:2238-44.
62. Voloboueva LA, Liu J, Suh JH. (R)-alpha-lipoic acid protects retinal pigment epithelial cells from oxidative damage. Invest Ophthalmol Vis Sci. 2005 Nov;46(11):4302-10.
63. Kowluru RA, Odenbach S. Effect of long-term administration of alpha-lipoic acid on retinal capillary cell death and the development of retinopathy in diabetic rats. Diabetes. 2004 Dec;53(12):3233-8.
64. Ihnat MA, Thorpe JE, Kamat CD, et al. Reactive oxygen species mediate a cellular “memory” of high glucose stress signaling. Diabetologia. 2007 Jul;50(7):1523-31.
65. Dos Santos JM, Kowluru RA. Role of mitochondria biogenesis in the metabolic memory associated with the continued progression of diabetic retinopathy and its regulation by lipoic acid. Invest Ophthalmol Vis Sci. 2011 Nov 11;52(12):8791-8.
66. Xiang G, Pu J, Yue L, et al. α-lipoic acid can improve endothelial dysfunction in subjects with impaired fasting glucose. Metabolism. 2011 Apr;60(4):480-5.
67. Haritoglou C, Gerss J, Hammes HP, et al. Alpha-lipoic acid for the prevention of diabetic macular edema. RETIPON Study Group. Ophthalmologica. 2011;226(3):127-37.
68. Kowluru RA, Kanwar M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr Metab (Lond). 2007 Apr 16;4:8.
69. Sajithlal GB, Chithra P, Chandrakasan G. Effect of curcumin on the advanced glycation and cross-linking of collagen in diabetic rats. Biochem Pharmacol. 1998 Dec 15;56(12):1607-14.
70. Gupta SK, Kumar B, Nag TC, et al. Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. J Ocul Pharmacol Ther. 2011 Apr;27(2):123-30.
71. Steigerwalt R, Nebbioso M, Appendino G, et al. Meriva, a lecithinized curcumin delivery system, in diabetic microangiopathy and retinopathy. Panminerva Med. 2012 Dec;54(1 Suppl 4):11-6.
72. Rema M, Pradeepa R. Diabetic retinopathy: an Indian perspective. Indian J Med Res. 2007 Mar;125(3):297-310.
73. Kowluru RA, Zhong Q, Santos JM, et al. Potential beneficial effects of carotenoids on diabetic retinopathy. Program Number: 4598, Association for Research in Vision and Ophthalmology (ARVO), Seattle, WA, May 8, 2013.

