2023 Pharma IssueIn the March issue of Review of Optometry, experts provide exciting updates on incoming dry eye treatments, oral steroid prescribing protocols and advancements in presbyopia management. Plus, learn how to fight insurance denials for new meds. Check out the other articles featured in this issue:
|
A broader range of product choices is becoming available to consumers across all facets of life, and optometry is certainly no exception. New technologies and treatments in eye care are developed every year, and 2023 may be the annus mirabilis for developments in presbyopia management.
Current presbyopia correction strategies already have a whole gamut of options for patients and eyecare providers to choose from, as corrective lenses in one form or another have always done the job well enough.1 We’re still in the very early days of pharmaceutical options for near vision correction but the category is set to blossom soon, with newer options offering different active ingredients and mechanisms of action. Let’s review several agents in the pipeline that may work their way to the public in the near future.
The Trailblazer
Vuity, approved in late 2021, has made its mark as the sole pharmaceutical agent available to the public as a form to treat presbyopia.2 How has the drop worked for patients since its approval and how does it fare in terms of efficacy?
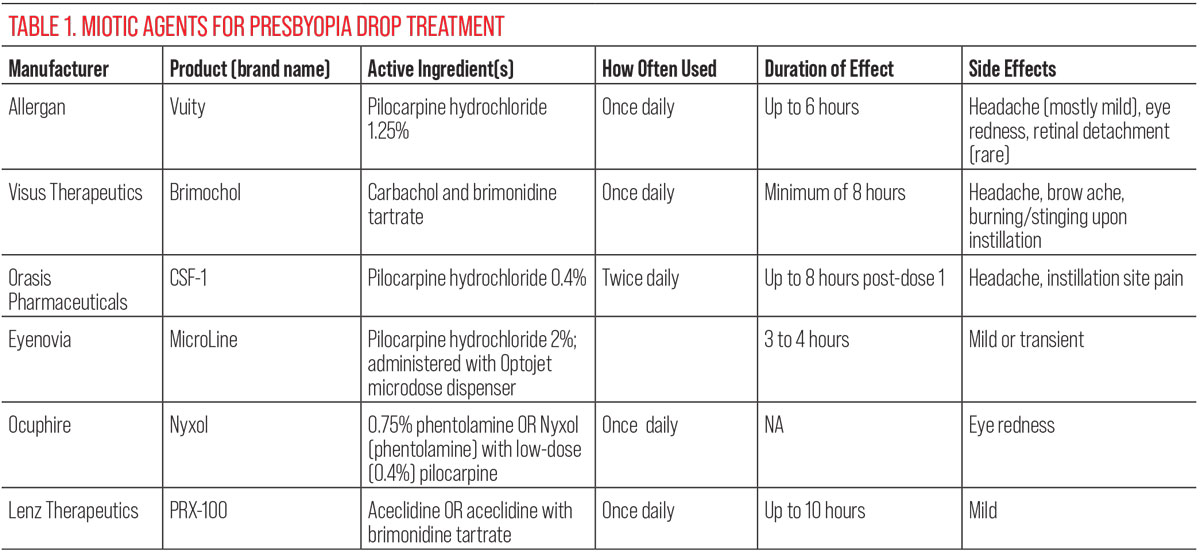
Produced by Allergan (now a part of AbbVie), Vuity’s active ingredient is a pilocarpine hydrochloride ophthalmic solution at 1.25% concentration.3 While pilocarpine has decades of familiarity to eye doctors as a now-outdated therapy for glaucoma, what makes Vuity different is Allergan’s use of a pH buffer (Allergan calls it pHast) that rapidly adjusts to the ocular surface pH and increases the drug’s bioavailability.4
Bisant Labib, OD, a professor and associate dean of optometric special programs at The Eye Institute at Salus University, reminds us how pilocarpine works on the eye in order to contextualize Vuity. “The action would stimulate parasympathomimetic effects, acting on those receptors of the ciliary body and iris sphincter, causing some degree of miosis to increase clarity up close.” This type of parasympathomimetic action on the eye creates a pinhole effect, allowing the decreased size of the pupil to achieve greater depth of focus and subsequently improving a patient’s near visual acuity.
As with any product to be the first of its kind, it’s not without shortcomings. The most notable include the duration of effect, potential side effects and cost. Allergan states that its drops are to be administered once daily in the morning. But with only about a six-hour duration—many anecdotal reports describe briefer periods—the effect can wear off and become inconvenient for users who are doing more throughout the day.3
Mile Brujic, OD, a partner of Premier Vision Group in Northwest Ohio, notes that, in his practice, he’s seen anywhere from four to 10 hours of effectivity, depending on the patient. Milton Hom, OD, of Azusa, CA echoes that sentiment, advising that you have to pick the right patients for this type of drop. He emphasizes that patients should have distance vision corrected to make the drops work more effectively. Dr. Brujic cites success not only with emmetropic presbyopes, but also with contact lens wearers who became presbyopic, multifocal users, uncorrected low myopes and uncorrected low astigmatism patients.
Another detriment to success is the potential side effects. While the most commonly occurring ones (>5%) are headache and eye redness, more troubling ones can occur. Dr. Hom highlights that because pilocarpine causes the ciliary muscles to contract, patients also complain of brow ache, as this mechanism can cause the brow to contract, too. Ciliary contraction is the same mechanism that can contribute to the rarer but much more serious side effect of vitreoretinal problems such as retinal detachment. A few such cases have been reported in patients using Vuity.
While inadequate duration of effect and potential side effects may not negatively affect every patient, price is a more pervasive stumbling block. At $79 for a one-month supply, the therapy is an investment, especially since it’s an out-of-pocket expense to patients.5
Dr. Brujic believes the impact of price is relative. “I think it totally depends on the patient, what the expectations are and what they’re interested in paying for it,” he says. “At the current dosing, you’re really looking at Vuity being essentially $2.50 per use. That’s what it really boils down to. Some people would say, ‘No, that’s not worth it to me.’ Other people say, ‘Yeah, it’s more than worth it.’”
Chris Wroten, OD, a partner at the Bond-Wroten Eye Clinics in Louisiana, has seen some patients embrace Vuity at launch, but didn’t see a significant increase in prescription fill rate even when the manufacturer temporarily cut the price per bottle in half. As he puts it, “Those who like it have filled it, but for others, it would appear that cost may not have been the only barrier to adoption. We all know there’s a certain percentage of patients who don’t like taking eye drops in general, so perhaps that is another barrier.”
A slew of next-generation products are hoping to leap these hurdles. Let’s review what’s being proposed.
 |
|
Dr. Hom notes that use of an eye drop for presbyopia may be worth the out-of-pocket cost for some if they don’t use it regularly but rather only in certain social or recreational occasions. Photo: Getty Images. Click image to enlarge. |
Dynamic Duo
Some investigational presbyopia therapies use two active ingredients in hopes of overcoming the limitations of pilocarpine alone. Brimochol, currently in development by Visus Therapeutics, doesn’t use pilocarpine at all. Instead, its active ingredients are carbachol, a cholinergic agent, and brimonidine tartrate, an alpha-2 agonist. The two active agents are supposed to complement each other in their effects.
“They’re making the pupil constrict as well as blocking the pupil dilator,” explains Dr. Hom. Dr. Brujic goes into more detail, outlining how brimonidine reduces pupils dilation by reducing the effect of the dilator muscle while carbachol acts on the sphincter muscles. As a result, “they seem to be having this long-lasting effect with a single dose.”
Like Vuity, Brimochol is supposed to be administered once daily, and Visus claims its product will work for a minimum of eight hours.6 The drug is currently in Phase III clinical trials with two underway: BRIO-I and BRIO-II.7
The company’s Phase II VIVID study documented a minimum of 83% of subjects achieving the three-line endpoint of improvement in binocular near visual acuity under mesopic conditions, while one line of distance vision was not lost after one hour. The same endpoint was met in a minimum of 82%, 52% and 35% of subjects after three, seven and nine hours, respectively.8
The most common side effects or adverse events (>5%) were similar to those of Vuity. These included headache and brow ache, as well as temporary burning or stinging upon drop instillation.8
Also like Vuity, carbachol likely acts in a way similar to pilocarpine through causing a degree of miosis, increasing near clarity, Dr. Labib notes.
Less is More
Other companies are taking a different approach to improve efficacy, opting for a lower strength pilo but more frequent instillation rather than doubling down on active ingredients. That’s the plan for CSF-1, a low-dose pilocarpine drop being developed by Orasis Pharmaceuticals. Unlike Vuity’s once-daily dosing, this version of pilocarpine was administered twice daily in its Phase III clinical trials, NEAR-1 and NEAR-2.
At only 0.4% concentration (about one-third that of Vuity), the drops displayed good efficacy in the trials. Across both studies, 40% and 50% of participants demonstrated a three-line or more gain with no loss of one line or more in distance visual acuity one hour after the first dose and one hour after the second dose.9 At day 15, this improvement occurred as early as 20 minutes and up to eight hours after the first dose.9
Adverse events included headache in 6.8% of participants and instillation site pain in 5.8%. Moderate adverse events were present in 2.6%.9
Understandably, the lesser adverse events are likely due to the decreased concentration of active ingredient used to achieve the same effect. As Dr. Hom explains, “When you reduce the concentration down, what you’re doing is reducing the risk of adverse events. What you want to do is achieve efficacy. But on the flip side of that, you want to use the lowest dose possible, because the higher dose will give you higher efficacy, but will also introduce more adverse events. So, you want to get the minimum dose you possibly can get and achieve efficacy.”
This thought process seems to be working for Orasis, as the company announced in February that the FDA accepted its New Drug Application for review. The agency has assigned a Prescription Drug User Fee Act goal date of October 22 of this year.10
 |
| Click image to enlarge. |
Presbyopia Spray
Much different from any of the other options, at least in terms of drug delivery method, Eyenovia’s MicroLine solution is meant to be dispensed through an aerosol dispenser it calls Optojet.11 Essentially making the liquid spray into the eye, this delivery method of pilocarpine offers better efficacy because of the administration type, its developers claim.
“It sprays into the eye, and it does it through a screen, so it has very small particles, making it like a mist,” Dr. Hom elucidates. “A regular drop will deliver so many milliliters or microliters of the drug, but this spray will imprint the cornea with a much smaller amount, so you get better penetration. That’s the reason why they can administer a lower concentration. He also mentions that the lower dosage additionally should lead to fewer adverse events.
Dr. Brujic brings up the point that this delivery system offers a solution for those who might have problems administering regular eye drops, making it more usable for some.
Eyenovia has released results from its Phase III clinical trial, VISION-2, about MicroLine’s efficacy. A 2% pilocarpine “micro-array print formulation,” as it’s called, was used and met its primary endpoint, the company says. The micro-array is a tool allowing for cells, proteins, DNA or polymers to be printed in complex patterns at high speeds.12 This approach allowed patients to achieve a 15-letter or more improvement of near visual acuity with less than a five-letter loss in distance acuity in low light conditions two hours post-administration.13
Adverse events were reportedly low, with fewer than 3% of patients experiencing mild or transient symptoms, possibly because the way the device works.13
Something Old, Something New
Combining pilocarpine with a novel agent is a further treatment possibility. Ocuphire’s Nyxol is another product with a unique active ingredient: 0.75% phentolamine ophthalmic solution. The company’s Phase II VEGA-1 trial tested both options of Nyxol with low-dose pilocarpine (0.4%) and Nyxol alone.14
For the group that was administered both phentolamine and low-dose pilo, 61% improved greater than three lines in near vision after one hour, compared to the placebo at 28%. This group also met the standard of less than five letters of distance vision loss.15
Since phentolamine is an alpha-adrenergic antagonist, it prevents or reverses pupil dilation, explains Dr. Brujic. He further explains that “it doesn’t necessarily work directly on the sphincter.” In addition to using the phentolamine, the additional low-dose pilocarpine allows the lower dose of pilocarpine to act with the alpha-adrenergic antagonist, resulting in complementary effects.
The Nyxol plus pilocarpine solution displayed full efficacy by 30 minutes and extended near vision improvement for at least six hours in the VEGA I trial. Some subjects even experienced a sustained reduction in pupil diameter over at least 18 hours.15
Unlike with many other presbyopia drops, no instances of headache, brow ache or blurry vision were reported in the VEGA-1 trial. However, there was mild and transient eye redness observed in less than 5% of participants.15
In a recent developmental milestone, Ocuphire announced in January that a first patient has been enrolled for the Phase III trial, VEGA-2.16
 |
|
The availability of presbyopia eyedrops allows even patients who rely on progressive lenses to occasionally get a boost in near vision if desired. Photo: Getty Images. Click image to enlarge. |
Killer Combo
There’s no shortage of new agents being tested as alternatives to pilocarpine. One final presbyopia drug in the near-term pipeline that fits this description is PRX-100. Currently being developed by Lenz Therapeutics, this drop’s active ingredient is aceclidine, a cholinergic agonist. This agent acts similarly to other agents that are cholinergic agonists but, interestingly, aceclidine seems to be more specific to just activating just the sphincter muscle with less activation of the ciliary muscle, Dr. Brujic points out.
He continues that there “apparently isn’t a lot of activity in the area of the ciliary muscle, making it so the muscles aren’t active. As such, it doesn’t have that level or that layer of cross-reactivity that we would expect to see with the other cholinergic agonists.” He does make sure to point out that there is a question surrounding this mechanism. That is: is there an additional level of safety conferred when ciliary muscles are left inactivated?
With this type of agent, Lenz was able to advance PRX-100 past Phase II. A clinical trial named INSIGHT included two formulations; LNZ100 (aceclidine) and LNZ101 (aceclidine + brimonidine). Both test formulas of the drug achieved a three-line or greater improvement of near visual acuity without loss of one or more distance visual acuity lines at one hour. LNZ100 experienced this result in 71% of patients and LNZ101 in 56%.17
Both groups maintained this result at the 10-hour mark, displayed at a rate of 37% in the LNZ100 group and 48% in LNZ101. Related to this result was the observed average pupil size staying within the range of 1.5mm to 2mm for the extent of the 10 hours.17
Lenz reported only low rates of mostly mild adverse events. With all these data, Phase III trials are to begin soon.
Where They Stand
After getting a sense of how each drop differs from the others, as well as their similarities, let’s consider how they might stack up outside of clinical trials and hard data points. With only one approved agent thus far, much of the sentiment is necessarily speculative at this point. But one good indication is how likely doctors are to prescribe or recommend these drops in their own practice, and why.
Dr. Labib believes presbyopia drops in general are better suited for people who are not doing near work all the time. “I don’t think that drop use is going to be the same as throwing on a pair of glasses that’s tailor made for you and lasts longer than six to eight hours. But if you’re just trying to get by and have some clarity at near, as well as distance, like if you’re a basketball coach or hairdresser who isn’t looking up close all the time, it’s going to be very different.” Ultimately, though, she believes the decision is going to come from the patient’s desire, lifestyle, amount of near work and how much extra near focus they’ll need.
She also considers age a factor to bear in mind if she were to prescribe any presbyopia drops, advising it may help more for younger presbyopes. The well-known and largely inevitable process of lens thickening with age makes the structure less prone to change shape and subsequently raises the bar for the drops to provide the same effectivity in more advanced presbyopia. As such, medical therapies may be a more useful treatment option only in emerging presbyopes.
 |
|
With near vision tasks more prominent these days, given the ubiquity of digital device use, more patients will be amenable to hearing about new options that can improve their lives. Photo: Getty Images. Click image to enlarge. |
Dr. Wroten comments on what patient personality might find drops appealing, as well as who might have the ideal ocular status. “Certainly, it’s got to be someone who doesn’t mind putting a drop in their eye every day. At least with pilocarpine-based drops, patients need to be aware of the potential for a slight headache, at least initially, as well as possibly a slight darkening of their vision. If they are aware of those side effects, which may be temporary, and can get past those, it seems to be most readily adopted in our patient base by those who reach presbyopia without requiring much correction for distance vision. That has been the sweet spot so far in our practice, but I look forward to trying the other presbyopia drops in the pipeline.”
He warns that some patients should be excluded from using presbyopia therapies, such as high myopes, because of the potential impact the active ingredients may have on the retina.
Dr. Brujic similarly points out that patient selection is “so dependent upon the individual’s wants, what they’re looking for and also what their current prescription is, because sometimes people just aren’t candidates for drops based on their prescription.” But for those who are a better fit, he sees no problem in prescribing the drops.
Dr. Hom believes the pharmaceutical therapies for presbyopia can be used in combination with other treatments, making it a flexible option for patients rather than a rigid all-or-nothing choice. “There’s no reason why you have to use it all the time. You can use it part time with whatever else you’re using. If you want to use them before putting on contact lenses or use them to do computer work, then they definitely work as an option.”
Worth the Cost?
If the going rate is $79 a bottle per month and supposing the newer therapies are priced comparably (actual prices have not been disclosed yet), this is a significant price to pay for the average person looking to manage presbyopia, potentially for several years, depending on the viability of the option for their eyes. All doctors have different, unique takes on whether the somewhat steep price is worth it to customers, but they agree on one point: as more presbyopia drops become approved and available to the public, competition should drive prices down.
“I think it’s still finding its place in the market and will be adopted by a subset of eligible presbyopes desiring independence from contact lenses and glasses, but for our colleagues concerned with losing a significant percentage of optical sales, I don’t foresee that happening,” Dr. Wroten says. “I think this gives our patients additional options, which is always a good thing, and can be additive with existing options.”
Dr. Labib has a different perspective, thinking that the drops target exactly who they’re supposed to: a regular contact lens wearer who doesn’t want to wear glasses at all. “I feel like they’re targeting the right people—the people who don’t want to wear glasses are the ones who are shelling out the money for multifocal lenses, so they would probably be the same people who would be amenable to spending money on an eye drop.”
Dr. Hom points out it may be worth the cost for some if they don’t use it regularly, but instead opt for using the drops in certain social or recreational occasions.
The general consensus from all doctors was that the cost will be worth it to some patients and others not. It really depends on the patient’s expectations and what they value in a treatment.
On the Horizon
What does the future of presbyopia drops look like? These doctors offer insight that establishes where drops started and where they might go.
Dr. Wroten looks ahead to the future of drops, hopeful to see potential alternative drug delivery options, like an extended-release option that could be incorporated into a drug-eluting contact lens, extending efficacy and eliminating the need to instill eye drops. If the patient did require distance correcting contacts, this could allow them to stay in a monofocal design. “Getting the effect of the medication without having to put a drop in could certainly be very attractive to patients,” he posits. Another extended-release option could be a punctal plug delivery system that also bypasses the need for drop instillation.
Dr. Brujic takes a similar approach to looking at alternative technologies, but not specifically for how to improve presbyopia drop administration. Instead, he hopes “we get to a place where we’re exploring alternative mechanisms other than reducing pupil size. I hope we start getting to the root cause, which is lens inelasticity. The current drops are increasing depth of focus through reduced blur circles, but it’s not functionally making the lens do anything differently. The lens is still inactive. We’re just strategically providing a different way to provide more functionality to presbyopes.”
This may be the case as presbyopia drops become more and more mainstream. Dr. Hom mentions that he was recently reading Walter Isaacson’s biography of Steve Jobs and a quote stuck out to him that he thinks applies to the state of presbyopia drops. Originally said by hockey legend Wayne Gretzky and often quoted by Steve Jobs during his life, a forward-thinking person should “skate to where the puck is going, not where it’s been.” Dr. Hom believes optometry must heed that advice, too.
Drs. Chris Wroten and Bisant Labib have no financial disclosures.
Dr. Milton Hom: Allergan/AbbVie, Bausch Health, Novartis, Sun Pharma, Kala Pharma, Tarsus Pharma, Hovione Scientia, Silk-Tech, Sydnexis, Topcon, Eyenovia Bio, Laboratories Thea, Aurinia Pharma, Eyevance Pharma, Surface Pharma, Nevakar, Visus Therapeutics, Aperta Biosciences, Astareal, Azura Ophthalmics, Aldeyra Therapeutics, FAMY Group, Bruder Health, Kowa, Ocuphire.
Dr. Mile Brujic has as no financial or proprietary interest in any of the products discussed. He has received honoraria in the past two years for speaking, writing, participating in an advisory capacity, research or meeting support from: Apellis, ABB Optical, Alcon Laboratories, Aldeyra, Allergan, Art Optical, Avellino, Bausch + Lomb Health, Contamac, CooperVision, CSEye, Dompe, Horizon Therapeutics, Johnson & Johnson Vision Care, Kala, Lenstech, Notal Vision, Novartis, Optovue, Oyster Point, Quidel, RVL, Sun Pharma, Tangible Science, Santen, Visus, Walman Optical and Zea Vision.
1. Boyd K. What is presbyopia? American Academy Ophthalmology. www.aao.org/eye-health/diseases/what-is-presbyopia. November 22, 2022. Accessed Feburary 9, 2023. 2. About Vuity. Allergan. www.vuity.com/about-vuity. Accessed February 9, 2023. 3. Vuity (pilocarpine HCl ophthalmic solution) 1.25%, the first and only FDA-approved eye drop to treat age-related blurry near vision (presbyopia) is now available [press release]. Abbvie. news.abbvie.com/news/press-releases/vuity-pilocarpine-hci-ophthalmic-solution-125-first-and-only-fda-approved-eye-drop-to-treat-age-related-blurry-near-vision-presbyopia-is-now-available.htm. December 9, 2021. Accessed February 9, 2023. 4. Reddy V, Coney JM, Thulasi P, Ahmad S. Vuity. EyeWiki. eyewiki.aao.org/vuity. Last updated February 12, 2023. Accessed February 15, 2023. 5. Cost and savings. Allergan. www.vuity.com/cost. Accessed February 15, 2023. 6. Brimochol. Visus Therapeutics. www.visustx.com/brimochol. Accessed February 15, 2023. 7. Visus Therapeutics initiates Phase III pivotal trials of Brimochol PF for the treatment of presbyopia [press release]. Visus Therapeutics. www.biospace.com/article/releases/visus-therapeutics-initiates-phase-3-pivotal-trials-of-brimochol-pf-for-the-treatment-of-presbyopia. March 22, 2022. Accessed February 15, 2023. 8. Visus Therapeutics announces positive topline clinical data from Phase II VIVID study of Brimochol for the treatment of presbyopia [press release]. Visus Therapeutics. www.businesswire.com/news/home/20211130005414/en/visus-therapeutics-announces-positive-topline-clinical-data-from-phase-2-vivid-study-of-brimochol-for-the-treatment-of-presbyopia. November 30, 2021. Accessed February 15, 2023. 9. Orasis Pharmaceuticals. Orasis Pharmaceuticals announces positive Phase III topline results of novel eye drop candidate, CSF-1, for the treatment of presbyopia [press release]. Cision. www.prnewswire.com/news-releases/orasis-pharmaceuticals-announces-positive-phase-3-topline-results-of-novel-eye-drop-candidate-csf-1-for-the-treatment-of-presbyopia-301529894.html. April 21, 2022. Accessed February 15, 2023. 10. Orasis Pharmaceuticals. FDA accepts Orasis Pharmaceuticals’ New Drug Application for CSF-1 for the treatment of presbyopia [press release]. Cision. www.prnewswire.com/news-releases/fda-accepts-orasis-pharmaceuticals-new-drug-application-for-csf-1-for-the-treatment-of-presbyopia-301750185.html. February 21, 2023. Accessed February 22, 2023. 11. Pipeline. Eyenovia. eyenovia.com/pipeline/presbyopia. Accessed February 15, 2023. 12. Microarray. BioDot. www.biodot.com/applications/microarray. Accessed February 24, 2023. 13. Eyenovia. Eyenovia announces positive results from VISION-2 Phase III study of MicroLine as a potential on-demand treatment for presbyopia [press release]. GlobeNewswire. www.globenewswire.com/en/news-release/2022/10/20/2538243/0/en/eyenovia-announces-positive-results-from-vision-2-phase-3-study-of-microline-as-a-potential-on-demand-treatment-for-presbyopia.html. October 20, 2022. Accessed February 15, 2023. 14. Ocuphire Pharmaceuticals. Safety and efficacy of Nyxol with pilocarpine eye drops in subjects with presbyopia. US National Library of Medicine. clinicaltrials.gov/ct2/show/NCT04675151. Last updated June 22, 2022. Accessed February 15, 2023. 15. Ocuphire’s VEGA-1 Phase II trial in presbyopia meets primary and secondary endpoints [press release]. Ocuphire Pharmaceuticals. www.ocuphire.com/news-media/press-releases/detail/344/ocuphires-vega-1-phase-2-trial-in-presbyopia-meets. June 30, 2021. Accessed February 15, 2023. 16. Ocuphire Pharma announces first patient enrolled in VEGA-2 pivotal Phase III trial of Nyxol in presbyopia [press release]. Ocuphire Pharmaceuticals. www.ocuphire.com/news-media/press-releases/detail/394/ocuphire-pharma-announces-first-patient-enrolled-in-vega-2. January 9, 2023. Accessed February 15, 2023. 17. Lenz Therapeutics announces positive topline data from Phase III INSIGHT trial of LNZ100 and LNZ101 to treat presbyopia [press release]. Lenz Therapeutics. lenz-tx.com/2022/10/lenz-therapeutics-announces-positive-topline-data-from-phase-2-insight-trial-of-lnz100-and-lnz101-to-treat-presbyopia/. October 18, 2022. Accessed February 15, 2023. |


