2023 Pharma IssueIn the March issue of Review of Optometry, experts provide exciting updates on incoming dry eye treatments, oral steroid prescribing protocols and advancements in presbyopia management. Plus, learn how to fight insurance denials for new meds. Check out the other articles featured in this issue:
|
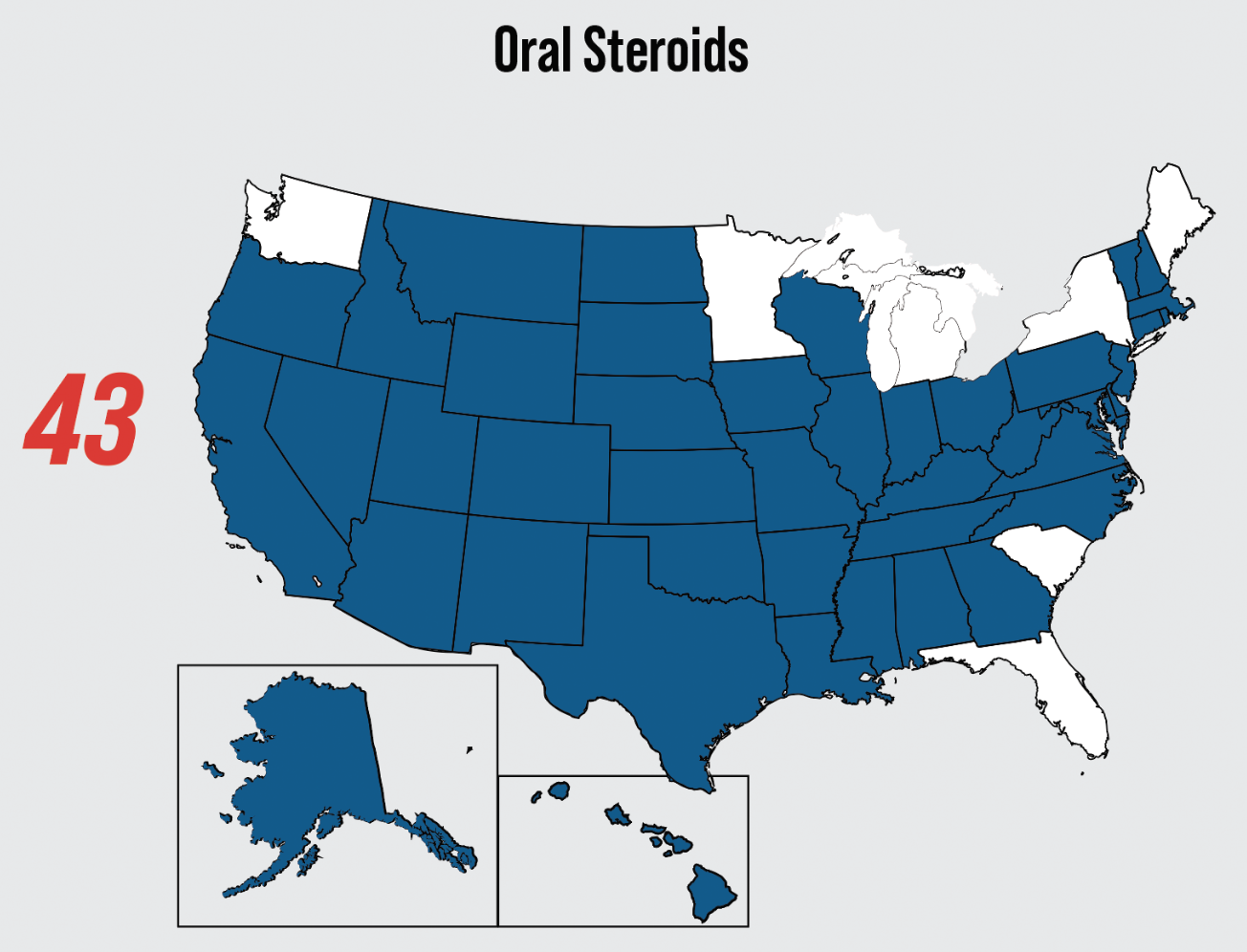
With the expanded scopes of practice in optometry across the country, optometrists currently have the legal authority to prescribe oral steroids in 43 of 50 states (Figure 1).1 Prescribing oral steroids for ocular pathologies can be intimidating due to the possibility of adverse systemic effects. However, the anti-inflammatory properties of corticosteroids can not only improve a patient’s severe anterior or posterior segment inflammatory event but can also be some of the most important vision-saving medications in neurological ocular events.
Here, we’ll review the mechanism of action, most common uses in eye care, side effects and management of oral steroids.
Mechanism of Action
To gain confidence in prescribing oral corticosteroids, it is imperative to have a clear understanding of how these medications work to control the body’s inflammatory response. Steroids function to inhibit the inflammatory cascade and prevent the damaging effects that severe ocular inflammation can cause, including scarring and neovascularization. These important medications work best in treating acute inflammatory processes, as opposed to chronic inflammation, which is better controlled with immunomodulators.2
Corticosteroids can be divided into two categories: glucocorticoids and mineralocorticoids. While both are 21-carbon structures synthesized in the adrenal cortex in response to ACTH (adrenocorticotropic hormone), each has distinct functions. Glucocorticoids function in the inflammatory pathway by binding to steroid receptors helping control the effects of stress on the body. Naturally occurring glucocorticoids include cortisol and cortisone. Synthetic glucocorticoids include prednisone, methylprednisolone, dexamethasone, fluorometholone and others. Mineralocorticoids bind to similar types of receptors and work to regulate water and electrolyte balance in the body. An example of a mineralocorticoid is aldosterone.3 Steroids are metabolized in the liver via the cytochrome P450 pathway.3
 |
|
Fig. 1. This map displays the 43 US states where optometrists are currently authorized to prescribe oral steroids.1 Click image to enlarge. |
Corticosteroids affect the immune and inflammatory responses at many points along their pathways. Steroids have been shown to function at both molecular and cellular levels to suppress inflammation and its effects. First, corticosteroids easily penetrate cell membranes and bind to specific steroid-binding protein receptors in the cytoplasm, creating a steroid-receptor complex. This complex travels to the nucleus, where it binds to chromatin and initiates the production of mRNA that codes for the proteins and enzymes that need to be synthesized in response to the steroid.3 This is important because these cellular and molecular changes lead to the steroid’s control over the cardinal signs of inflammation, including pain, erythema, edema and heat.3
There are numerous mechanisms of action that allow steroids to successfully control an inflammatory event. Edema is reduced by the constriction of blood vessels, limiting their permeability. This lessens the amount of fluid, protein and inflammatory cells that can leak into the extracellular space.3 Steroids also help decrease cytokine activity. Cytokines are the inflammatory cells that signal to the body that an immune response is needed, flooding the site with inflammatory cells.2 Steroids work to prevent these cytokines, such as polymorphonuclear cells, from clinging to vascular endothelium.3 Steroids also inhibit phospholipase A2, which prevents the breakdown of arachidonic acid into prostaglandins and leukotrienes. These medications also prevent inflammatory cells such as neutrophils and macrophages from entering the affected tissues. Together, these mechanisms help to reduce pain, erythema and heat in the affected area. Lastly, and importantly, steroids quell fibroplasia or scarring.3
Oral Steroid Use in Eye Care
Since their introduction into ocular treatment in 1951 to manage both anterior and posterior chamber inflammatory pathologies, oral steroids have since been used to treat a wide range of eye conditions.2 The two oral steroids that are typically prescribed are prednisone and methylprednisolone. All steroids have a basic structure of 21 carbons in four rings. Prednisone and prednisolone have an additional double bond in ring A. Methylprednisolone is created by the methylation of carbon 6 in ring B. It also has a slightly greater anti-inflammatory effect.3 Prednisone is an oral medication only, while methylprednisolone can be given intravenously or orally.
Below are some of the most common etiologies optometrists will encounter that may require oral corticosteroid therapy.
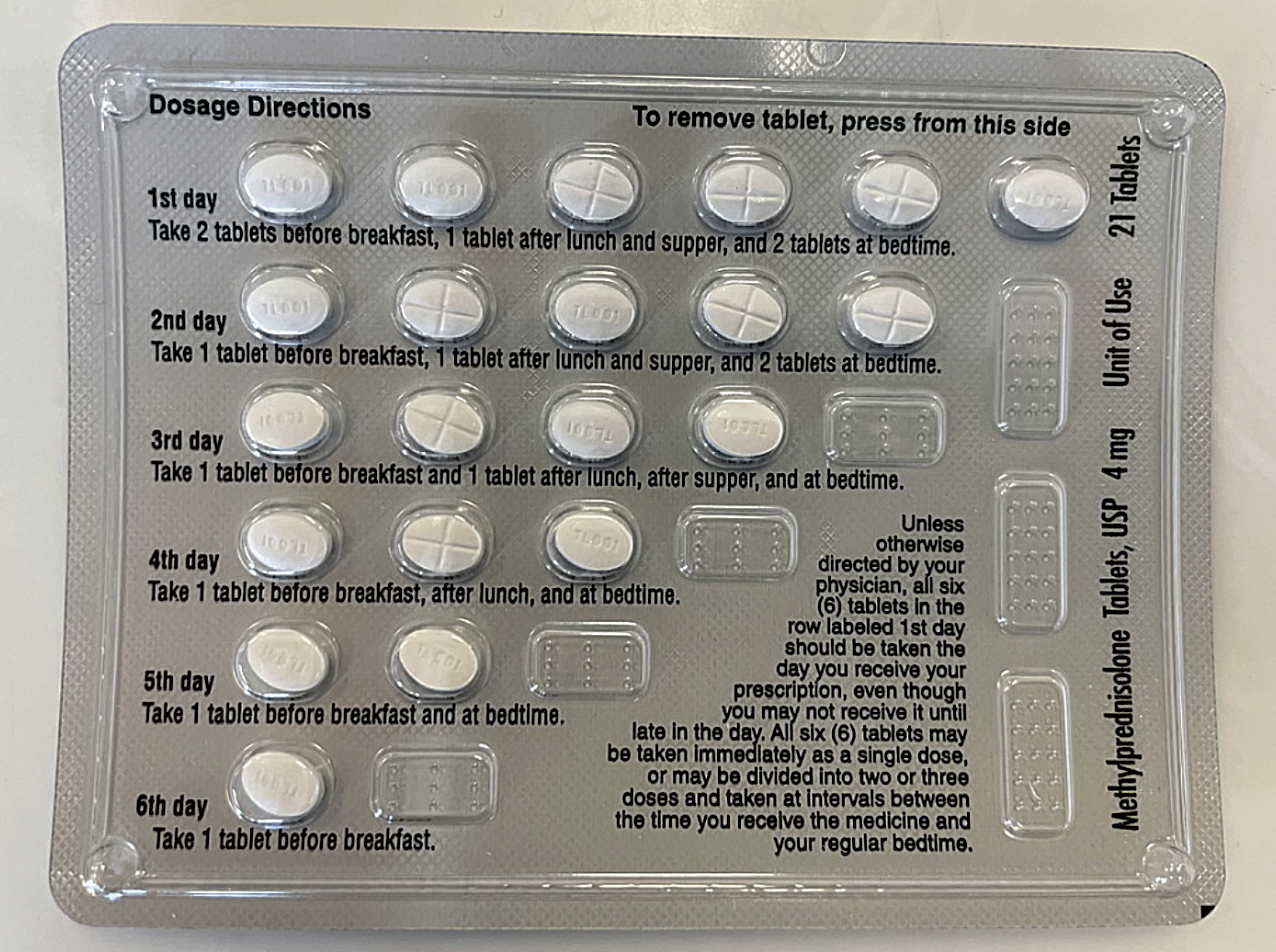
Periocular dermatitis. For patients with inflammation of the eyelids and adnexa secondary to allergic dermatitis, methylprednisolone, commonly ordered as a 4mg Medrol Dosepak, is beneficial and convenient for ocular inflammation that is superficial (Figure 2).2 A Medrol Dosepak is a pre-packaged dose of methylprednisolone that has the patient take six 4mg tablets on day one, tapering one tablet per day until all are taken. During the summer months, superficial ocular inflammation is most commonly seen with dermatitis secondary to allergy to poison ivy. Prescribing oral steroids in a Medrol Dosepak is one of the more convenient and economical options for patients. It delivers a higher dose of steroids on day one with a built-in tapering schedule that is easy to follow.
 |
|
Fig. 2. Medrol Dosepak (4mg) showing easy-to-follow instructions for patients with a built-in taper. Photo: Jessica Steen, OD. Click image to enlarge. |
Bell’s palsy. When patients present with new-onset Bell’s Palsy, a short-term course of oral steroids can calm the inflammation of the seventh cranial nerve. The treatment outcome will be best if treatment is initiated within three days of the start of the facial nerve palsy. The recommended dose is prednisone 60mg to 80mg/day for one week. Treatment with a steroid at this dosage in short duration is likely to be easily endured without many side effects.4 Antiviral agents may also be considered in addition to prednisone, but the benefit has not been well proven in literature.6
Thyroid eye disease (TED). In the treatment of this condition, previously known as Graves’ ophthalmopathy, steroids are used to decrease inflammation or overcrowding by reducing soft tissue edema within the orbit. They are prescribed to decrease the intraorbital pressure and are only effective during new or quickly progressing ophthalmopathy, not with chronic disease.2 Oral prednisone should be prescribed at 60mg to 100mg/day. Once improvement occurs, attempt to taper the daily dose to the least daily amount of prednisone that maintains the positive results.5 Tapering the dosage by 5mg to 10mg/week is the recommended rate, but you may need to stop the taper and increase the steroid dosage if symptoms worsen again.2 If there is no improvement within four to six weeks of oral prednisone, alternative treatments should be explored, including referral to oculoplastics for consideration of Tepezza (teprotumumab-trbw).
Non-infectious uveitis. If a non-infectious undifferentiated uveitis is not responding to topical steroids or sub-Tenon steroid injections, systemic steroids may be required. If the uveitis is bilateral, systemic steroids are often the preferred second line of therapy to topical instead of injections. In non-infectious undifferentiated intermediate uveitis that does not respond to topical or sub-Tenon steroid injections, oral steroids should be considered. Oral steroids are often needed for posterior uveitis. Oral prednisone dosed at 40mg to 60mg daily for four to six weeks, followed by a slow taper, is the recommended treatment course.6
Scleritis. For scleritis, topical steroids are not effective. In cases of diffuse, nodular and necrotizing scleritis, systemic steroids may be required. The recommended way to prescribe oral prednisone is for one week at 60mg to 100mg daily, then begin a taper to 20mg daily for two to six weeks, followed by a continued slow taper.6 Scleritis that is recurrent in nature or due to underlying autoimmune disease should be referred to rheumatology for consideration of other immunosuppressive therapies.
Giant cell arteritis. In patients suspected to have giant cell arteritis, emergent testing of erythrocyte sedimentation rate (ESR), C-reactive protein and platelets should be performed. If giant cell arteritis is diagnosed, systemic steroids should be initiated as soon as possible. The initial treatment is methylprednisolone 250mg intravenously every six hours for 12 doses followed by oral prednisone 80mg to 100mg daily while awaiting the results of a temporal artery biopsy, which is the most sensitive and specific test for giant cell arteritis.2,6
The initiation of treatment should not be withheld while awaiting the temporal artery biopsy. It is recommended that temporal artery biopsy should be done within one week of the initiation of treatment with systemic steroids. Once the results of the temporal artery biopsy have come back with positive findings, the patient needs to remain on the prednisone 1mg/kg/day.6 The original dosage of the steroid is continued until the ESR returns to normal and symptoms improve. This is followed by a slow taper with monitoring of the ESR and symptoms with each dosage reduction or at least monthly. If the ESR begins to rise or symptoms recur, prescribers should revert patients to the previous increased prednisone dosage.
When treating giant cell arteritis with oral steroids, consider comanagement with a neurologist or neuro-ophthalmologist due to the dosage and length of treatment and the host of potential side effects. Treatment should continue for at least six months to a year.6
Idiopathic orbital inflammatory syndrome. Also known as orbital inflammatory pseudotumor, the suggested treatment for diagnosed cases is oral prednisone 1mg to 1.2mg/kg/day as the initial dose. Patients who respond to the oral steroids should remain at the original dosage for several days. Then, a slow taper to 40mg/day for two weeks is recommended, followed by a continued unhurried taper below 20mg/day completed through several weeks.6
Optic neuritis. Another neuro-ophthalmic disorder that can require steroid therapy is optic neuritis. A pulsed dose of IV methylprednisolone should be given at 1g/day for a total of three days. The patient can then be converted to oral prednisone dosed at 1mg/kg/day for 11 days, after which the medication can be tapered over four days, with 20mg the first day and 10mg on days two to four.6 This should be initiated within 14 days of the onset of vision loss. The Optic Neuritis Treatment Trial found that oral prednisone should never be used as the first-line treatment due to the potential for an increased chance of recurrence. The trial also found that steroids only shortened the amount of time until vision is fully recovered. Another treatment choice is simply observation, as visual acuity will return to the same level as if it was treated by steroids.6
Managing Side Effects
Nearly every tissue in the human body has steroid receptors, which may lead to adverse effects in many different organ systems. These adverse effects are also dependent upon the strength of the dose and the length of the treatment. Patients taking higher dose steroid treatment for chronic conditions like giant cell arteritis are more likely to have systemic side effects than patients taking a short course of lower-dose steroids for an etiology such as contact dermatitis secondary to poison ivy.
Let’s review the most common side effects of oral steroids and how to best approach them.
Gastrointestinal. Various GI side effects can arise from oral steroid use, including gastritis, peptic ulcers and bleeding in the GI pathway. If steroids are combined with NSAIDs, the risk of gastrointestinal effects increases two-fold.7 To protect the stomach, it is highly recommended for the patient to be placed on an anti-ulcer medication such as a proton pump inhibitor (available as OTC Prilosec or Prevacid) or a histamine type 2 (H2) receptor blocker (available as OTC Pepcid).1,6 In addition, be sure to educate patients to not take NSAIDs such as ibuprofen or naproxen while on oral corticosteroid therapy.
Adrenal insufficiency. When oral steroids are taken for a prolonged period, the adrenal system ceases making the natural cortisol used in the daily functioning of the body. The hypothalamic-pituitary-adrenal (HPA) axis is the pathway that controls endogenous cortisol production and becomes inactivated when a patient takes steroids over time. The amount of endogenous corticosteroids created by the HPA-axis is equivalent to 7.5mg of prednisolone daily (or about 8mg of prednisone daily).8 When high-dose steroids are discontinued abruptly, acute adrenal insufficiency may occur.12 Acute adrenal insufficiency can be life-threatening with symptoms including hypotension, severe weakness, acute abdominal pain with nausea and vomiting, fatigue and lethargy.9
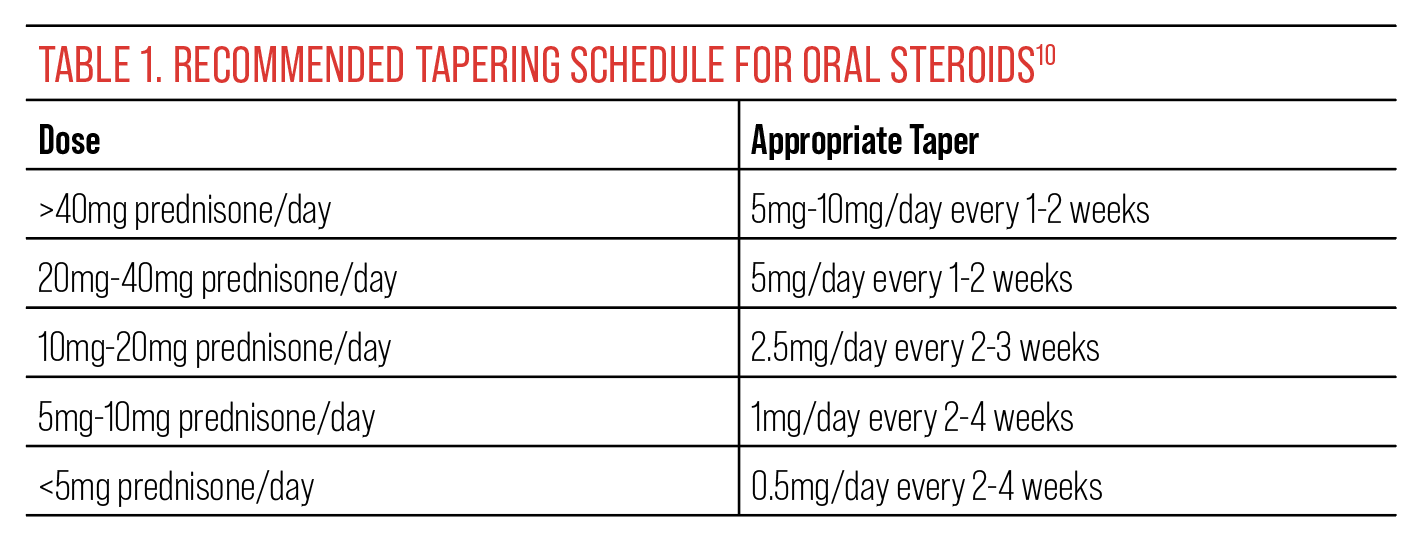
To prevent acute adrenal insufficiency, tapering of steroids is imperative. HPA-axis suppression is not likely to occur in patients who have received glucocorticoids for less than three weeks in duration.10 In patients treated longer than three weeks, HPA-axis suppression has likely occurred, meaning the body is no longer synthesizing its own cortisol. Tapering the patient slowly will allow the HPA-axis to regain function and prevent the recurrence of any inflammation from withdrawal of the corticosteroid.10 Due to this, it is recommended that patients on therapy courses longer than two weeks should be tapered. This is slightly less than the three-week mark to ensure all patients who have HPA-axis suppression are treated safely. See Table 1 below for recommended tapering schedules.
 |
| Click image to enlarge. |
Endocrine. Oral systemic steroids can cause hyperglycemia by stimulating the liver to create glucose and by reducing the rate at which glucose is used.11 The higher the steroid dosage, the higher the risk for increased blood glucose. Patients with diabetes may experience elevation of blood glucose levels and will need comanagement with their primary care provider to help control blood glucose levels.7
Long-term use of corticosteroids may also lead to Cushing syndrome, which is the redistribution of body fat that occurs in response to hypercortisolism. The three primary sites of increased body fat are the face (moon face), neck (buffalo hump) and the trunk (truncal obesity). These effects tend to be dose-dependent and can occur within the first two months of steroid therapy.7,10 Cushing syndrome can be treated by the slow taper of the corticosteroid treatment. Weight gain without Cushing syndrome may occur as well due to steroids causing increased appetite.7
Central nervous system. Oral glucocorticoid treatment can lead to numerous side effects of the central nervous system. In short-term therapy, the most reported effect is mood elevation. Though this effect doesn’t sound all that threatening, it can be more severe in some patients who report mania, insomnia and restlessness.11,12 One of the only circumstances when a patient should abruptly have steroids either discontinued or the dose significantly reduced is in the case of steroid-induced psychosis that does not respond to antipsychotic medications.10 Depression may also occur with patients on a longer course of steroid therapy.11,12 Most of the time, these symptoms are minor and will resolve after oral steroid discontinuation.7
Cardiovascular. Hypertension can be seen in patients on oral steroid treatment, especially in patients who have underlying heart or kidney disease. This is due to corticosteroids promoting the retention of fluid due to changes in electrolyte and water balance.7,11 Steroid-induced hypertension has been observed in up to 20% of Cushing syndrome patients and is largely dose-dependent.7 Comanaging the patient with their primary care provider, internist or cardiologist is recommended to ensure blood pressure remains in therapeutic range while on long-term steroid therapy.
Ophthalmological. Ocular side effects of oral steroids include cataracts, elevated intraocular pressure (IOP) increasing the risk of glaucoma and in rare cases, exophthalmos.7 The most common form of cataract seen with systemic steroid treatment is posterior subcapsular. The incidence of cataracts is heavily dependent on dose and duration.2,3
It is common for optometrists to monitor IOP closely when topical steroids are used in the eye, but it’s also important when patients are undergoing systemic therapy. Systemic therapy has been shown to elevate IOP that can potentially lead to optic nerve damage and visual field loss.3 IOP-lowering medications can be initiated to prevent this damage while a patient is undergoing long-term steroid therapy. It’s wise to ask all autoimmune disease patients about any steroid therapy, past or present.
Steroid IOP response is much more common in patients with an existing diagnosis of glaucoma. For these patients especially, IOP should be closely monitored throughout treatment with oral steroids.
Lastly, another instance when steroids should abruptly be discontinued, or dose quickly reduced, is a patient with a herpesviral corneal ulcer. Oral steroid treatment could lead this to perforate causing irreparable damage.10
Skin, skeletal and muscle. Skin thinning and bruising can occur with steroid treatments. Glucocorticoid induced myopathy can also occur, leading to muscle weakness and fatigue.7 Osteoporosis is a common side effect of systemic steroids that also increases a patient’s risk for bone breakage. Steroids decrease calcium absorption in the GI tract while increasing osteoclast activity to break down bone.12 This is another reason the dosage and length of steroid treatment should be kept to as little and short as possible.
Patients at higher risk of these effects due to fragility or elderly status should be monitored more closely for osteoporosis or treated prophylactically. OTC calcium and vitamin D supplementation should be recommended to anyone at risk or anyone who will likely require steroid treatment lasting three months or longer. For these patients, calcium intake should be 1000mg to 1200mg/day and vitamin D intake should be 600 IU to 800 IU per day.13
Immune system. While a patient is undergoing oral steroid treatment, their immune system activity is reduced. Hence, any new infection or injury may result in serious consequences. Quick antimicrobial therapy is important during this time to prevent any opportunistic infection from causing further issues with the patient’s health.11 Wound healing is also impaired, so close monitoring is especially required for patients with diabetes, cancer, those who are elderly or those on other immunosuppressive medications.7
Contraindications to Steroid Use
Peptic ulcer disease, tuberculosis, active infections and psychosis are all contraindications to the prescribing of oral corticosteroids.6 Patients need to be screened for symptoms of psychosis, which may include a history of depression, delirium, hallucination, confusion or distress.7 Steroids are also contraindicated during pregnancy.6 Children should be prescribed steroids with caution due to inhibition of growth with steroid medications.12
Corticosteroids may also cause interactions with other medications the patient may be taking, and it will be important to review their current medications prior to prescribing. Caution is advised with nonsteroidal anti-inflammatory agents or anticoagulants, which may need prophylaxis to avoid gastroduodenal toxicity.7
While not a contraindication, patients with comorbidities—including diabetes mellitus, poorly controlled hypertension, significant cardiovascular issues (i.e., atherosclerotic disease and arrhythmias), cataracts, glaucoma, peptic ulcer disease and osteoporosis should be prescribed steroids judiciously.7 It is wise to consult with the patient’s primary care provider or internist when considering oral steroid therapy for these individuals.
Conclusion
Oral steroids offer patients multiple advantages, such as the ease of administration and potential ability of the drug to calm whole-eye inflammation.2 Yet, the potential for systemic side effects can make prescribing oral steroids daunting. To help reduce incidence of these adverse events, the dosage for systemic oral steroids should be tailored to each individual patient, prescribing the lowest dosage that delivers control of the inflammatory event.
Always remember to recommend preventive ulcer medications and calcium supplements for long-term steroid use. Understanding the mechanism of action of oral steroids, the most common uses in eye care and the side effects/contraindications to oral steroids makes prescribing them when indicated much less intimidating.
Dr. Burress is a staff optometrist at the Jack C. Montgomery VA Medical Center in Muskogee, OK, and a fellow of the American Academy of Optometry. She also serves as an executive member of the Oklahoma Association of Optometric Physicians Congress Committee and as a chairsperson for ACOE residency accreditation visits.
Dr. Turner is a staff optometrist and residency and externship coordinator at the Jack C. Montgomery VA Medical Center. She is also a fellow of the American Academy of Optometry and serves as adjunct faculty for several colleges of optometry. Dr. Burress and Dr. Turner have no financial interests to disclose.
1. Nalley C. Prescribe oral meds like a pro. Review of Optometry. Published August 15, 2022. www.reviewofoptometry.com/article/prescribe-oral-meds-like-a-pro. Accessed January 11, 2023. 2. Bartlett JD, Jaanus KE. Clinical Ocular Pharmacology. Butterworth-Heinemann. 2008. 3. Albert DM, Miller JW, Jakobiec FA. Albert & Jakobiec’s principles and practice of ophthalmology. Saunders Elsevier. 2008. 4. Ronthal M, Greenstein P. Bell’s palsy: treatment and prognosis in adults. UpToDate. Published January 3, 2023. www.uptodate.com/contents/bells-palsy-treatment-and-prognosis-in-adults. Accessed January 11, 2023. 5. Davies TF, Burch HB. Treatment of thyroid eye disease. UpToDate. Published January 3, 2023. www.uptodate.com/contents/treatment-of-thyroid-eye-disease?search=graves+ophthalmopathy&source=search_result&selectedTitle=2~65&usage_type=default&display_rank=2#H472030470. Accessed January 11, 2023. 6. Gervasio KA, Peck TJ, Fathy CA, Sivalingam MD. The Wills Eye Manual: office and emergency room diagnosis and treatment of eye disease. Lippincott Williams & Wilkins. 2022. 7. Saag KG, Furst DE. Major side effects of systemic glucocorticoids. UpToDate. Published July 7, 2022. www.uptodate.com/contents/major-side-effects-of-systemic-glucocorticoids?search=graves+ophthalmopathy&topicRef=7827&source=see_link. Accessed January 11, 2023. 8. Joseph RM, Hunter AL, Ray DW, Dixon WG. Systemic glucocorticoid therapy and adrenal insufficiency in adults: a systematic review. Semin Arthritis Rheum. 2016;46(1):133-41. 9. Puar TH, Stikkelbroeck NM, Smans LC, Zelissen PM, Hermus AR. Adrenal crisis: still a deadly event in the 21st century. Am J Med. 2016;129(3):339.e1-9. 10. Furst DE, Saag KG. Glucocorticoid withdrawal. UpToDate. Updated July 28, 2022. www.uptodate.com/contents/glucocorticoid-withdrawal/print?search=steroid+taper&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1. Accessed January 11, 2023. 11. Brunton LL, Knollmann Björn C. Goodman & Gilman’s the pharmacological basis of therapeutics. McGraw Hill. 2023. 12. Ritter J. Rang and Dale’s pharmacology. Elsevier. 2020. 13. Rosen HN, Saag KG. Prevention and treatment of glucocorticoid-induced osteoporosis. UpToDate. Published August 30, 2022. www.uptodate.com/contents/prevention-and-treatment-of-glucocorticoid-induced-osteoporosis?search=graves+ophthalmopathy&topicRef=7988&source=see_link. Accessed January 11, 2023. |


