 |
Sudden-onset, unilateral, painless vision loss is certainly a cause for patient and doctor concern as it may indicate acute retinal ischemia (ARI). Transient monocular vision loss (TMVL) is the most common symptom of ARI with an incidence of approximately 14 per 100,000.1 Ophthalmic exams on TMVL patients usually lack significant findings and sequelae. In contrast, ARI from artery occlusions produce permanent visual acuity, visual field loss, or both.1 Though treatment is mostly futile in restoring vision in these cases, patients should still be emergently referred for a stroke work-up.2
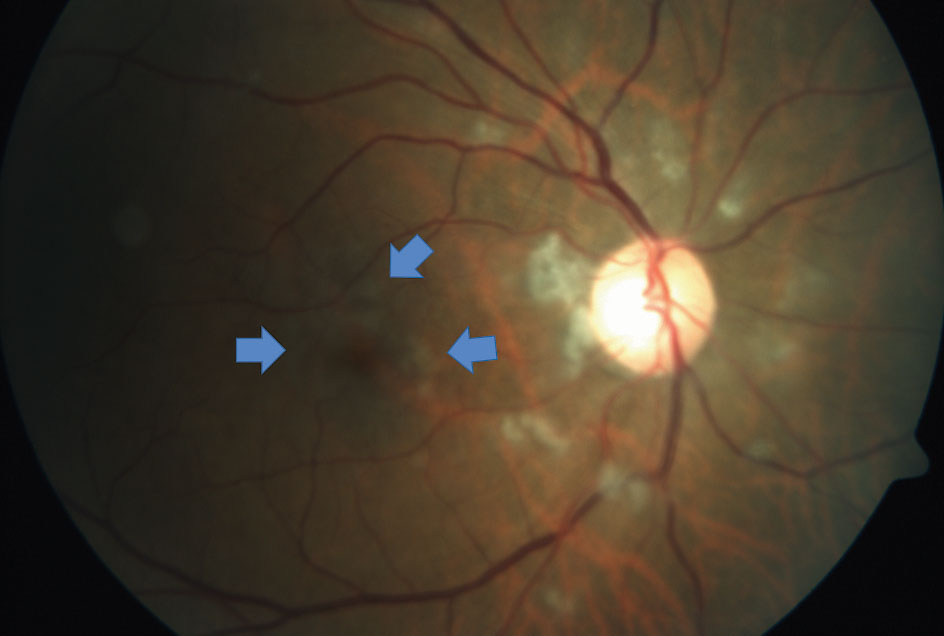 |
| Fig. 1. This right eye fundus photograph documents the presence of cotton-wool spots surrounding the optic nerve. More subtle signs of ischemia surround the fovea (arrows). Vessel caliber appeared mostly symmetrical with the unaffected eye. Click image to enlarge. |
The Patient
A 77-year-old African-American male presented with a complaint of constant black spots in his right eye for one week that did not change with eye movement. He also reported reduced vision in the right eye along with weakness on the right side since its onset. His best-corrected visual acuity was 20/20-2 OD with +0.75DS, and 20/20 OS with +0.25-0.50x105.
Pupils were equally round and reactive and without an afferent pupillary defect (APD). Both extraocular motilities and confrontation visual fields were unremarkable. Amsler grid was unreliable in the right eye due to problems fixating centrally. His medical history included anemia, hypertension and hyperlipidemia. His ocular history was positive for uncomplicated cataract surgery with posterior chamber intraocular lenses (PC-IOL) OU in 2014. His medications included allopurinol, hydrochlorothiazide, levetiracetam, potassium chloride and sildenafil.
Anterior segment examination showed a clear PC-IOL OD and a PC-IOL OS with temporal posterior capsular opacification in the left eye. Intraocular pressures were 10mm Hg OD and 11mm Hg OS with Goldmann applanation tonometry. A dilated fundus exam revealed 0.3 round cup-to-disc ratios with distinct margins in both eyes, paramacular retinal whitening in the right eye and cotton-wool spots surrounding the right nerve (Figure 1). The left eye was unremarkable. Same-day ancillary testing included optical coherence tomography (OCT-B) with angiography (OCT-A), and intravenous fluorescein angiography. A visual field was not performed due to the urgency from the above test results.
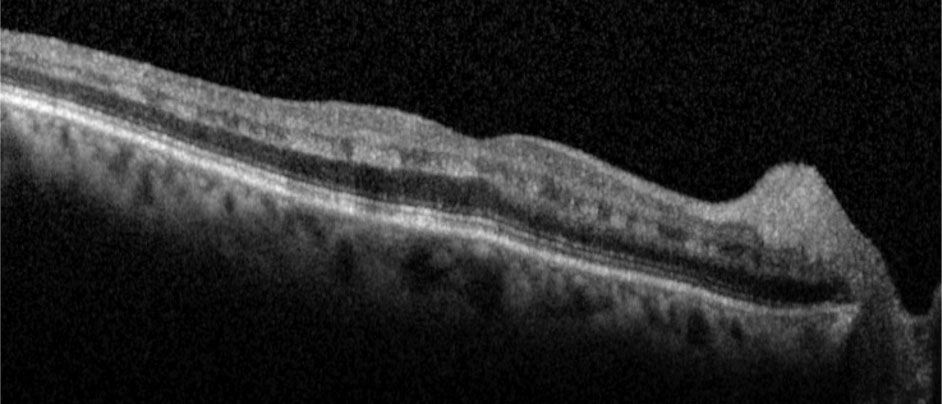 |
| Fig. 2. Hyperreflective bands in the inner nuclear layer consistent with paracentral acute middle maculopathy. Click image to enlarge. |
OCT-B detailed hyperreflective bands at the level of the inner nuclear layer consistent with paracentral acute middle maculopathy (PAMM) (Figure 2). OCT-A highlighted intereye asymmetrical superficial vessel density and choriocapillaris blood flow (Figures 3 and 4). The IVFA recorded an extended arm-to-retina time of 45 seconds with a significant delay of subsequent phases in the right eye (Figure 5). Auscultation of the right carotid was negative for a bruit.
The patient was referred to the emergency room with the eye care providers alerting doctors prior to his arrival. A magnetic resonance imaging scan was negative for any acute intracranial abnormalities. A computed tomography angiogram detected a stenosed right ophthalmic artery without significant carotid artery occlusion (Figure 6).
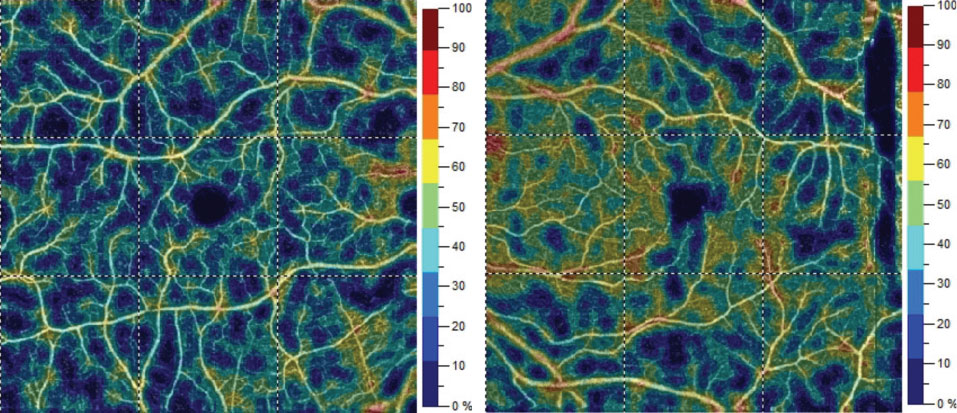 |
| Fig. 3. Color map representation of superficial vessel density percentages OD (left) versus OS (right). Comparative grid-based vessel density between the eyes varied from 2% to 15%. Click image to enlarge. |
Follow Up
ARI events are classic causes for sudden monocular vision loss and usually result from an embolic episode.1,2 From a vascular standpoint, unilateral visual disturbances often trace to the anterior cerebral circulation (i.e., the internal carotid artery, or ICA). Of significance to the optometrist is the first branch of the ICA—the ophthalmic artery—which subsequently divides to provide circulation to the optic nerve, choroid and retina.3
An occluded ophthalmic artery (OAO) produces profound ischemia and vision loss (light perception or no light perception) as both the retina and choroid are left devoid of circulation. This patient’s visual acuity (20/20-2) and pupil findings (no APD) are attributed to the partial vs. full OAO. In this case, hemodynamic disturbances unrelated to an embolus resulted in ocular ischemia. Hayreh explained this through his anatomical studies, which discussed perfusion pressure. He demonstrated that a stenosed ophthalmic artery with little or no ICA stenosis is primarily responsible for the fall in blood pressure in the ocular vascular bed.4
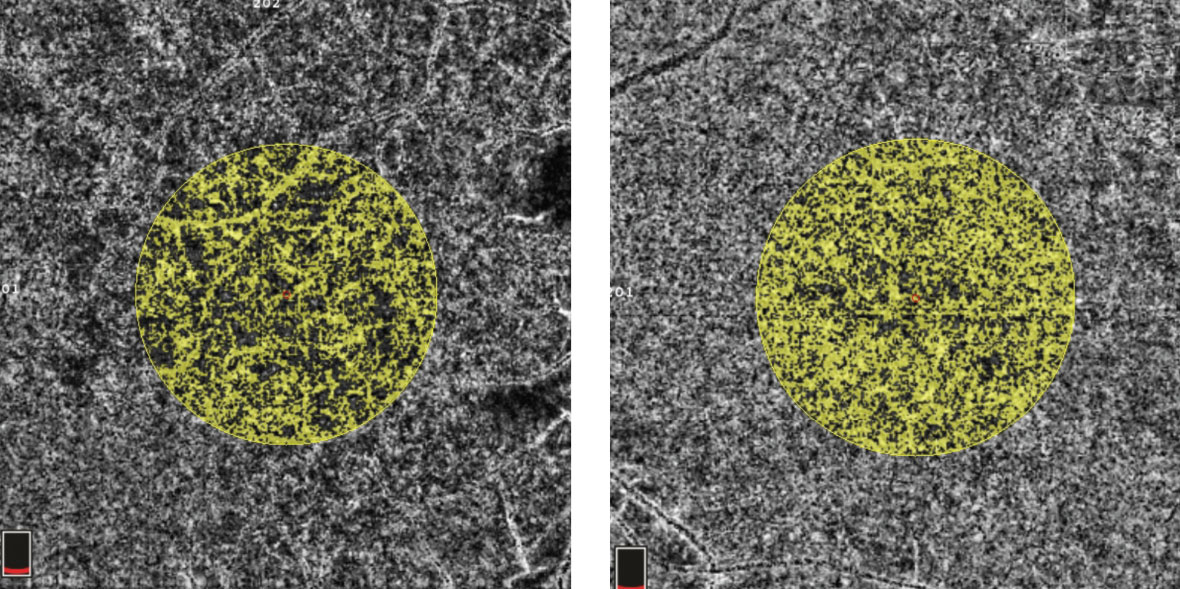 |
| Fig. 4. In this OCT-A image, the right eye (left) shows a choriocapillaris flow area of 3.763mm2 surrounding the fovea in an examiner-selected area of 7.114mm2. The left eye has an increased flow area of 4.686mm2 within the same area. Click image to enlarge. |
Clinically, a complete OAO lacks the “cherry red spot” seen in central retinal artery occlusions due to the lack of choroidal blood flow. Retinal vessel constriction and optic disc edema are also seen. Later in the disease, retinal pigment epithelium (RPE) alterations occur.5 The Almaric sign, which appears as triangular patches of retinal whitening during posterior choroidal artery occlusion, may be present.6 These patches mostly point toward the posterior pole or disc and result from infarction deep within the retina, which causes outer nuclear layer and RPE necrosis.6
The presence of hyperreflective bands seen on OCT-B in this patient is characteristic for PAMM, which affects the middle layers of the macula and indicates ischemia.7 These bands co-localize precisely with the intermediate and deep vascular plexi, which in tandem comprise the deep vascular plexus.8
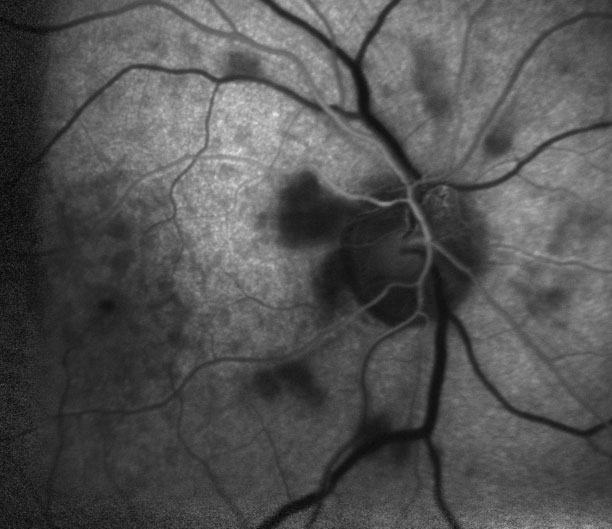 |
| Fig. 5. The depicted arterial phase of the fluorescein angiography occurred at three times that of a normal eye, which indicates delayed blood flow. |
Besides stenotic and embolic etiologies, OAO can also be due to hypercoagulable states or a vasculitis such as giant cell arteritis. Appropriate bloodwork should be ordered for these entities. Several authors report OAO following injection of cosmetic facial fillers such as autologous fat or hyaluronic acid.6,9 Optometrists should be aware of this given the rising popularity of these procedures. Others cite illicit drug use as an origin for OAO. Cocaine produces prolonged vasoconstriction and enhances platelet aggregation, while intravenous drugs often contain the filler talc, which can be potentially embolic.10
A lesser discussed condition called “Saturday night retinopathy” should also be considered with OAO. In this instance, OAO occurs with an acute rise in orbital cavity pressure by direct compression of the orbit when a patient falls asleep in a stuporous state following heavy alcohol or drug use.11
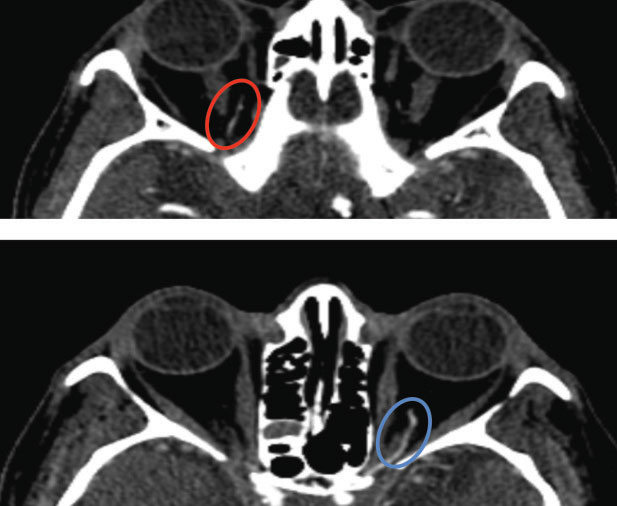 |
| Fig. 6. CT angiogram reveals a stenotic right ophthalmic artery (top, red oval) compared to the unaffected left ophthalmic artery (bottom, blue oval). |
Though proposed treatments fail at restoring vision in patients with ARI secondary to an arterial occlusion, both the National Stroke Association and the American Heart Association consider “retinal cell death attributable to ischemia” as a stroke equivalent.2 Recognizing an arterial occlusion as the cause for an ARI puts optometrists at the interventional front in these cases. This is significant, considering stroke is the fifth most common cause of death in the United States.12 Low vision services should also be discussed with patients who suffer visual loss that interferes with their activities of daily living.
1. Dattilo M, Newman NJ, Biousse V. Acute retinal arterial ischemia. Ann eye Sci. 2018;3(1):1-19. 2. Biousse V, Nahab F, Newman NJ. Management of acute retinal ischemia: Follow the guidelines! Ophthalmology. 2018;125(10):1597-1607. 3. Toma N. Anatomy of the ophthalmic artery: Embryological consideration. Neurol Med Chir (Tokyo). 2016;56(10):585-91. 4. Hayreh SS, Zimmerman MB. Ocular arterial occlusive disorders and carotid artery disease. Ophthalmol Retin. 2017;1(1):12-18. 5. Ko MK, Kim DS. Posterior segment neovascularization associated with acute ophthalmic artery obstruction. Retin J Retin Vitr Dis. 2000;20(4):384-88. 6. Lim S, Cheng CK, Li YH. Amalric triangular sign in a case of central retinal artery occlusion combined with posterior ciliary artery occlusion – Case report. Am J Ophthalmol Case Reports. 2018;11(October 2017):149-52. 7. Sarraf D, Rahimy E, Fawzi AA, et al. Paracentral acute middle maculopathy a new variant of acute macular neuroretinopathy associated with retinal capillary ischemia. JAMA Ophthalmol. 2013;131(10):1275-87. 8. Chen X, Rahimy E, Sergott RC, et al. Spectrum of retinal vascular diseases associated with paracentral acute middle maculopathy. Am J Ophthalmol. 2015;160(1):26-34. 9. Szantyr A, Orski M, Marchewka I, et al. Ocular complications following autologous fat injections into facial area: Case report of a recovery from visual loss after ophthalmic artery occlusion and a review of the literature. Aesthetic Plast Surg. 2017;41(3):580-4. 10. Gokoffski KK, Thinda S. Ophthalmic artery occlusion after cocaine use. J Emerg Med. 2015;49(1):61-2. 11. Malihi M, Turbin RE, Frohman LP. Saturday night retinopathy with ophthalmoplegia: A case series. Neuro-Ophthalmology. 2015;39(2):77-82. 12. Chancellor BK, Ishida K. New standards of care in ischemic stroke. J Neuro-Ophthalmology. 2017;37(3):320-31. |

