 |
A 74-year-old Hispanic female presented for a comprehensive eye exam complaining of blurry vision, worse in her left eye. She believed this was due to glue she had used previously to repair her eyeglass frames.
Her best-corrected visual acuity was 20/40 OD and 20/50 OS and her potential acuity pinhole (PAP) VA was 20/30 OD and 20/50 OS. Her slit lamp exam revealed mild blepharitis and conjunctival chalasis OU. She had 2+ nuclear sclerosis and trace cortical cataracts OD and 1+ NS with trace cortical cataract OS. Her dilated fundus exam of the right eye appeared normal and the left eye showed lesions in the macula (Figures 1 and 2).
An OCT was performed and is available for review (Figure 3). Humphrey Field Analyzer 10-2 testing showed central defects OU (Figures 4 and 5). Color vision testing with Ishihara plates was performed and was essentially normal (11/12 plates OD and 9/12 plates OS).
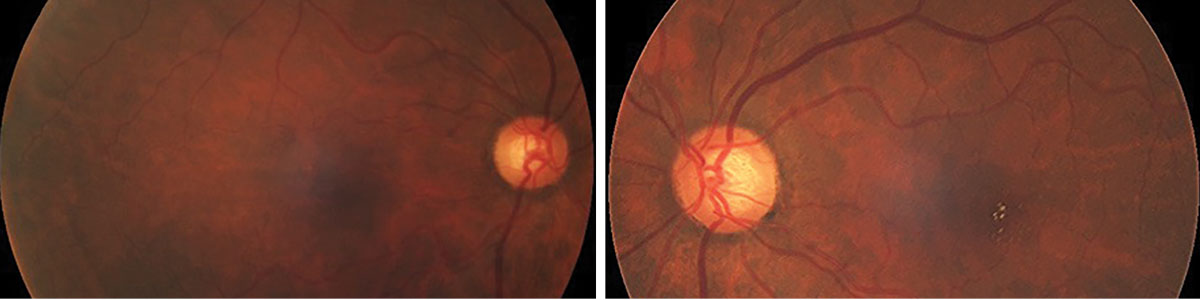 |
Figs. 1 and 2. Fundus photos of the right and left retina eyes. What do you see in her macula? Click image to enlarge. |
Take the Retina Quiz
1. What do the yellow lesions seen in the fungus photos of the left eye represent?
a. Exudates.
b. Emboli.
c. Drusen.
d. Crystalline deposits.
2. How would you interpret the OCT findings in our patient?
a. Normal.
b. Disruption of the IS/OS junction.
c. Focal superficial hyper reflective lesions.
d. Both b and c.
3. What is the correct diagnosis for our patient?
a. Idiopathic macular telangiectasia.
b. Tamoxifen toxicity.
c. Multiple evanescent white dot syndrome (MEWDS).
d. Cystinosis.
4. What is the most likely cause of the patients decreased PAP VA?
a. Crystalline deposits.
b. Disruption in IS/OS junction.
c. Macular edema.
d. Cataracts.
For answers, see below.
Diagnosis
There was no doubt our patient has nuclear sclerotic cataracts in both eyes, but is that the reason for her reduced acuity? In the right eye, the cataract was indeed worse and possibly consistent with her visual acuity (20/40), but the cataract in the left eye was clearly not as bad. We graded the nuclear sclerosis in the OS at only 1+, and though there were cortical lens changes, the cataract did not account for 20/50 visual acuity. So, is there something else going on with our patient?
Examination of her macula did provide some important clues. Though the macula in her right eye appeared normal, there were multiple intraretinal crystals present in the macula in her left eye. What’s more, there were also some interesting OCT findings that proved to be relevant. In both eyes, there were superficial hyperreflective retinal deposits and disruption in the inner segment-outer photoreceptor segment (IS/OS) junction. Interestingly, the findings in the right eye were not visible on funduscopy and only became apparent with OCT.
Given this finding, we went back through our patient’s medical history and recalled that she had a history of breast cancer and was being treated with tamoxifen 20mg daily for about three years. Therefore, a diagnosis of tamoxifen retinopathy was made.
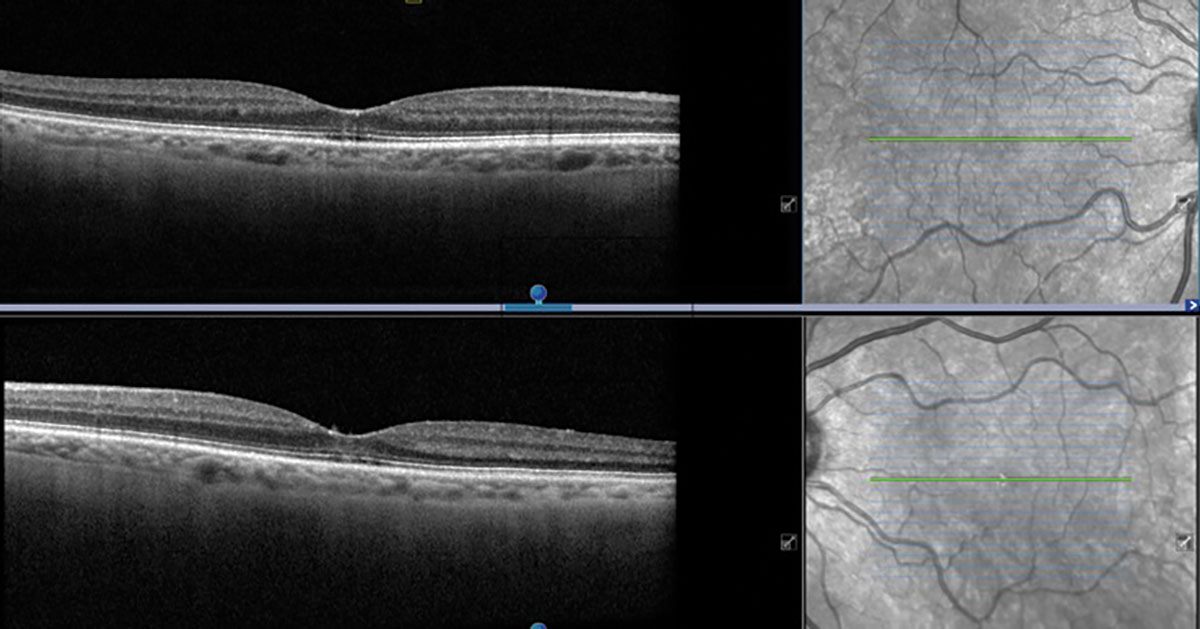 |
Fig. 3. Heidelberg OCT of the right and left eyes. Is this normal? Click image to enlarge. |
Discussion
Tamoxifen is an oral selective estrogen receptor antagonist that targets breast cancer cells and has the ability to penetrate the blood-retinal barrier.1 Case reports documenting tamoxifen toxicity exist from as early as 1994. Potential ocular manifestations of toxicity include a variety of corneal opacities, isolated instances of optic neuritis and varying changes to retinal layers. Retinal manifestations of tamoxifen toxicity reported thus far include intraretinal crystal deposition, which are thought to represent degenerated axonal material. Macular edema, retinal cavitation due to Müller cell disruption and disruption in the IS/OS junction have all been reported as well.2
As seen in the fundus photo, our patient appeared to have crystalline deposits in the left eye only until OCT later revealed that both eyes were affected. Because crystalline deposits are rarely visually significant, the IS/OS junction disruption in each eye is likely the cause for the decreased vision. Further, the reduced PAP visual acuity suggests that the decrease in best-corrected visual acuity cannot be completely attributed to the cataracts alone, at least in the left eye.
In several case reports, there has been evidence of partial recovery of the IS/OS junction and reversal of macular edema after discontinuing ramoxifen.3 However, crystals tend to remain within the retina for long periods of time.
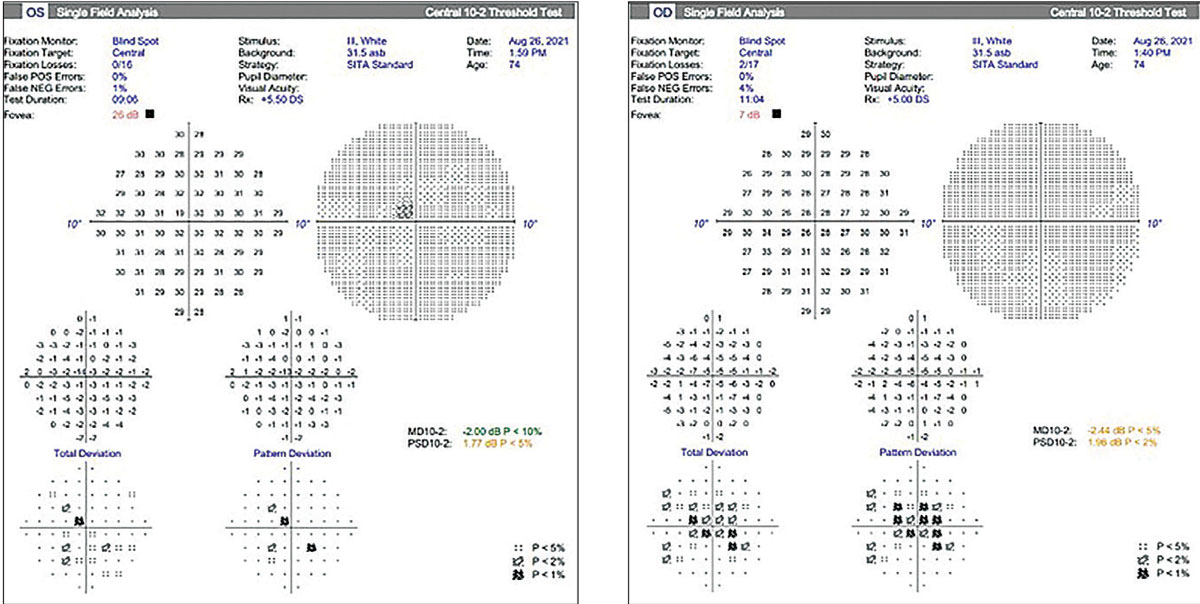 |
| Figs. 4 and 5. Standard Humphrey 10-2 visual fields were performed and show a central depression in both eyes. Does this represent artifact or is it a real defect? Click image to enlarge. |
While the exact mechanism of retinal toxicity is still unknown, previous studies involving human-derived RPE cells and mouse-derived photoreceptor cells have shown that tamoxifen may induce excess autophagy and cell death, as well as increased oxidative stress and zinc levels.1 Due to differing imaging modalities used, the incidence of retinal changes has a wide range reported in literature, from 1.5% to approximately 12%.4 Of note, studies that used OCT to identify retinopathy reported higher cases of retinopathy as compared to studies that solely used fundus photography or fundus exam. This may suggest that tamoxifen toxicity is in fact more common than earlier reports indicate.
Unlike with chloroquine toxicity, there is no established protocol for monitoring and screening patients for tamoxifen retinopathy. As demonstrated in this case, toxicity can manifest subclinically; therefore, it may be of value to obtain baseline ancillary testing on patients taking tamoxifen so that future changes can be identified. A retrospective analysis identified 30 of 251 patients with tamoxifen retinopathy, all of which were on a 20mg per day dose for two or more years.4 They also found that patients with a higher BMI and hyperlipidemia may be at increased risk.4 In contrast to earlier reports, this suggests that patients on a low dose of tamoxifen are still at risk for toxicity and may be more vulnerable if they possess these risk factors. Therefore, closer follow-up may be indicated than recommended in literature thus far.
Due to evident retinal changes that were seen in our patient, we consulted her oncologist to discuss alternative treatments and potential discontinuation of tamoxifen. Unfortunately, she did not return for her follow-up thereafter.
Retina Quiz Answers
1: d; 2: d; 3: b, 4; b
Dr. Dunbar is the director of optometric services and optometry residency supervisor at the Bascom Palmer Eye Institute at the University of Miami. He is a founding member of the Optometric Glaucoma Society and the Optometric Retina Society. Dr. Dunbar is a consultant for Carl Zeiss Meditec, Allergan, Regeneron and Genentech.
Dr. Siddiqui graduated from the Illinois College of Optometry and is completing her ocular disease residency at the Bascom Palmer Eye Institute.
| 1. Cho KS, Yoon YH, Choi JA, et al. Induction of autophagy and cell death by tamoxifen in cultured retinal pigment epithelial and photoreceptor cells. Invest Ophthalmol Vis Sci. 2012;53(9):5344-53. 2. Nayfield SG, Gorin MB. Tamoxifen-associated eye disease. A review. J Clin Oncol. 1996;14(3):1018-26. 3. Ghassemi F, Masoomian B, Khodabandeh A, et al. Tamoxifen induced pachychoroid pigment epitheliopathy with reversible changes after drug discontinuation. Int Med Case Rep J. 2020;13:285-9. 4. Kim HA, Lee S, Eah KS, Yoon YH. Prevalence and risk factors of tamoxifen retinopathy. Ophthalmology. 2020;127(4):555-7 |

