When a doctor discovers bilaterally elevated optic nerve heads (ONH), she or he faces a particular diagnostic challenge, especially when that patient is a child. Distinguishing congenital etiologies of optic disc elevation (known as pseudopapilledema) from true papilledema is imperative. True papilledema is a medical emergency. However, its incidence among the pediatric population is relatively low—even among patients who doctors initially suspect.1 According to one case series, three quarters of the pediatric patients referred for papilledema actually had a form of pseudopapilledema, or a variation of normal optic disc.1 Pseudopapilledema is a benign elevation of the ONH, associated with hypoplastic nerves, tilted nerves, crowded disc or optic nerve head drusen (ONHD).2
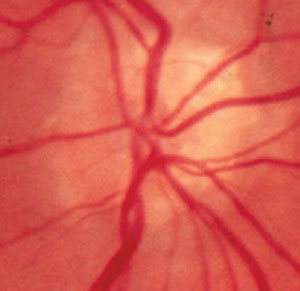 | |
| Fig. 1. A chalky, scalloped disc margin characteristic of ONHD is seen on the nasal portion of this optic disc. Note the early vessel branching and trifurcation as the blood vessels exit the nerve. |
ONHD are the most common cause of pseudopapilledema. The condition affects 0.4% of the pediatric population, with 75% of cases manifesting bilaterally.3-5 ONHD are refractile bodies composed primarily of calcium, deposited anterior to the lamina cribrosa. Since drusen may displace the neuroretinal tissue anteriorly, it may cause a false appearance of optic nerve swelling.6,7 Researchers speculate that smaller-than-average scleral canals may be susceptible to static axoplasmic flow, which causes dysfunction of metabolic capabilities of the axon and results in drusen deposits at the disc.2
Papilledema, on the other hand, is a true medical emergency indicated by bilateral swelling of the optic nerve due to increased intracranial pressure.2 This pressure is transmitted to the subarachnoid space surrounding the optic nerve (ON), causing increased pressure around the ON and resulting in blockage of axoplasmic transportation and edema of the nerve fiber layer (NFL).8 Causes of papilledema in the pediatric population may include, but are not limited to, Guillain-Barré syndrome, spina bifida, hydrocephalus, intracranial mass, trauma/subdural hemorrhage, meningitis, subdural venous thrombosis, arteriovenous malformation and idiopathic increase in intracranial pressure.9-11
This article reviews how to properly distinguish between pseudopapilledema, such as ONHD, and true papilledema, as well as how to use various diagnostic modalities to make the distinction.
| Table 1. Features of ONHD vs True Papilledema. | |||
| Key Features | Optic Nerve Head Drusen | True Papilledema | |
| Visual Symptoms | Transient vision loss (TVL) & Visual field defects (VFDs) can occur | TVL, double vision, and VFDs | |
| Headaches | Not associated with ONHD | If present are described as worse upon awakening &/or postural changes | |
| Neurological Symptoms | Not associated with ONHD | Tinnitus, vertigo, nausea/vomiting, peripheral neuralgias | |
| Optic Nerve Appearance | Elevation confined to disc, SVP present | Elevated swollen nerve, hyperemia, peripapillary vessel obscuration +/- flame shaped hemorrhages, cotton wool spots, +/- Paton’s linesSVP absent | |
| Vasculature | Anomalous branching pattern | Microvascular dilation | |
Funduscopic Evaluation
Although no set protocols exist for the evaluation of pseudopapilledema vs. true papilledema, a comprehensive eye exam with a dilated fundus evaluation and the use of fundus photography can pose great value.12 In some cases, characteristic funduscopic findings may help distinguish ONHD from true papilledema. The presence of a spontaneous venous pulse (SVP) is a significant finding which rules out true papilledema. However, an absence of SVP occurs in 20% of the normal population and, in some cases, the presentation of the SVP may be rather subtle.13 Furthermore, evaluation for the presence of SVP may be taxing, due to poor cooperation and fixation by pediatric patients.
Visible ONHD show observable calcifications within the disc in addition to a chalky, scalloped disc margin (Figure 1). However, visible calcifications are not always evident, since most ONHD in the pediatric population are buried. Although both true papilledema and ONHD will present with elevated disc margins, in cases of ONHD the elevation is confined to the disc. The disc margin may be indistinct and in some cases may possess a scalloped appearance. Elevated disc margins associated with ONHD manifest no vessel obscuration at the disc margin. Anomalous vascular branching patterns depicted as an increased number of retinal vessels, early branching or trifurcation or both (Figure 1) are key features of ONHD. Furthermore, a dark gray appearance deep in the peripapillary tissue may be visible in cases of ONHD (Figure 2).
In cases of true papilledema, vessel obscuration occurs due to swelling of the NFL, hence elevation expands into the peripapillary retina. The optic disc may be described as hyperemic associated with superficial microvascular dilations and telangiectasia (Figure 3). Paton’s lines, described as retinal folds or buckling, may also be present.8 More advanced stages of true papilledema manifest with peripapillary cotton wool spots and retinal hemorrhages.8 It is important to note that, although not common, vascular changes such as peripapillary retinal hemorrhages or vein occlusion can also occur in ONHD.14 In addition, patients with ONHD may be at risk for developing choroidal neovascular membranes.15
Symptoms such as diplopia, transient visual obscuration, headaches and tinnitus are observed with papilledema. Thirty-three percent of patients with true papilledema are asymptomatic.2
Although fundus photography, particularly stereo photos (Figure 2), can aid in the evaluation of the optic disc in pediatric patients who have difficulty remaining at the slit lamp, differentiating true papilledema from ONHD may be a challenge using only a static photo.2
Non-invasive Imaging Technologies
Doctors have several non-invasive imaging options for these situations.
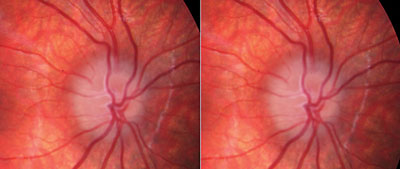 | |
| Fig. 2. Stereoscopic fundus photo of ONHD. Abnormal trifurcation is noted, as well as dark appearance deep in the peripapillary tissue. |
• B-scan Ultrasonography. B-scan is considered to have high sensitivity and specificity for the detection of ONHD.2,11 In the general patient, the optic nerve appears as a tubular structure with low homogenous reflectivity. Drusen appear as hyperreflective calcified bodies in the optic nerve and will continue to show increasing brightness even at a low gain. B-scan of true papilledema shows elevation of the optic disc with absence of hyperreflective calcified bodies.2 A crescent sign representing a ring of fluid around the optic nerve may also be noted in true papilledema.16 It can be difficult to identify deeply buried drusen on B-scan, as the hyperreflectivity is less evident than surfaced drusen. Furthermore, the absence of calcifications does not confirm true papilledema, since other causes of pseudopapilledema, such as a crowded disc, may still be present.
• Spectral-domain optical coherence tomography (SD-OCT). SD-OCT of ONHD will reveal an elevated disc with a characteristic “lumpy-bumpy” appearance. On the other hand, a nerve with true papilledema will reveal a smooth internal contour of the ONH with a characteristic hyporeflective “V” pattern in the subretinal space adjacent to the ONH.17,18 The “V” contour is presumed to be due to hydrostatic pressure from the ONH leaking into the subretinal space, resulting in an enlarged separation between the neurosensory retina and retinal pigmented epithelium (RPE).17,18 Investigators report that a small subretinal space also exists when ONHD are present; however, the subretinal space is smaller than that seen in true papilledema.19
Blood within blood vessels block the SD-OCT signal, casting a shadow onto the underlying tissues, which can easily be confused for the contoured appearance of ONHD. This is one reason why the SD-OCT image should be compared with ONH photos. Blood vessels are smaller and more superficial than drusen and have presence of reflective tissue completely encircling the blood vessel.7 Enhanced-depth imaging OCT can detect buried ONHD, as it can image deeper ocular structures than the SD-OCT.20
An increase in retinal nerve fiber layer (RNFL) thickness values can further aid in the evaluation of true papilledema. RNFL may appear to be unaffected or thinner in ONHD than in the age-related normative database, but are thicker in patients with true papilledema.18,21 However, with the use of SD-OCT alone, many early cases of true papilledema may look similar to ONHD. A recent case study using SD-OCT in the evaluation of ONHD and true papilledema found no difference in RNFL thickness between eyes with mild papilledema and buried ONHD.17 The concern lies in that potentially life- and sight-threatening conditions may be missed if one relies on a single modality test, as cases of ONHD can easily be confused with mild cases of papilledema.17
• Fundus autofluorescence (FAF). The calcific properties of drusen have inherent autofluorescent ability, thus ONHD will hyperfluoresce on FAF. Research shows this method is effective in diagnosing ONHD in pediatric patients.22 Remember, however, that autofluorescence of drusen is inversely proportional to its depth, which means deeply buried drusen may be difficult to assess using FAF.20 In true papilledema, the optic nerve swelling can extend into the surrounding tissue and mask the autofluorescence of the fundus, causing the appearance of an enlarged ONH.22
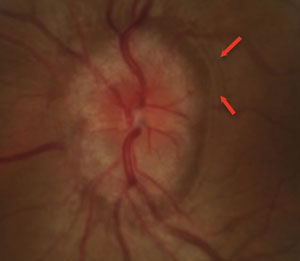 | |
| Fig. 3. Papilledema found in a pediatric patient presenting with microvascular dilation of the optic nerve, vessel obscuration and Paton’s line (red arrows). |
• Visual field testing. While ONHD is regarded as a benign cause of ONH elevation, functional changes, such as visual field defects, may be noted in rare cases. Visual field defects associated with ONHD include enlarged blind spots, localized focal defects, arcuate visual field defects or generalized constrictions. These may be present in 73% of visible drusen cases in contrast with only 36% of cases associated with buried drusen.23
As buried drusen begin to rise to the surface of the optic disc, compression of the neighboring axons can ensue, leading to cell apoptosis and, ultimately, progressive visual defects.22 This is more commonly noted in the second decade of life.24 Visual field testing can be used to monitor progression in cases of ONHD. Also, consider baseline testing in the early stages.5 Clinicians should take into account the maturity and attention span of the pediatric patient when considering visual field testing in the pediatric population. Most children older than eight years can perform a fairly reliable visual field test.
Referral
In any case where the diagnosis of pseudopapilledema is not confirmed, referral to a neuro-ophthalmologist is warranted. Radiological imaging, such as MRI or CT, followed by lumbar puncture (LP) may be necessary. An opening pressure of greater than 280mm H2O in the pediatric patient older than one year is highly suspicious for increased intracranial pressure.9
Although careful funduscopic evaluation of the optic disc can help differentiate pseudopapilledema from true papilledema, the pediatric population provides unique challenges. In the absence of associated intracranial pressure signs and symptoms, multimodal noninvasive ophthalmic imaging techniques may be used as effective tools to differentiate ONHD from true papilledema—thus, aiding in prompt referrals when necessary, while preventing unnecessary referrals for more invasive testing, and decreasing patient and parental anxieties.2,4
Dr. Ubhi is an optometric resident in primary care and pediatrics at Nova Southeastern University College of Optometry.
Dr. Shechtman is a professor of optometry at Nova Southeastern University College of Optometry, and an attending optometric physician at the Eye Institute and Diabetic/Macula Clinic.
Dr. Green is an optometric resident in pediatrics and binocular vision at Nova Southeastern University College of Optometry.
1. Kovarik J, Doshi P, Collinge J, Plager D. Outcome of pediatric patients referred for papilledema. Journal of AAPOS. 2015;19(4):344-8.2. Chiang J, Wong E, Whatham A, et al. The usefulness of multimodal imaging for differentiating pseudopapilloedema and true swelling of the optic nerve head: a review and case series. Clinical and Experimental Optometry. 2015;98(1):12-24.
3. Erkkila H. Clinical appearance of optic disc drusen in childhood. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1975;193(1):1-18.
4. Mehrpour M, Torshizi F, Esmaeeli S, et al. Optic nerve sonography in the diagnostic evaluation of pseudopapilledema and raised intracranial pressure: A cross-sectional study. Neurology Research International.2015:Article ID 146059.
5. Auw-Haedrich C, Staubach F, Witschel H. Optic disk drusen. Surv Ophthalmol. 2002;47(6):515-32.
6. Pineles S, Arnold A. Fluorescein angiographic identification of optic disc drusen with and without optic disc edema. Journal of Neuroophthalmology. 2012;(32)1:17-22.
7. Wester S, Fantes F, Lam B, et al. Characteristics of optic nerve head drusen on optical coherence tomography images. Ophthalmic Surg Lasers Imaging. 2010;41(1):83-90.
8. Killer H, Jaggi G, Miller N. Papilledema revisited: is its pathophysiology really understood? Clin Experiment Ophthalmol. 2009;37(5):444-7
9. Avery R. Interpretation of lumbar puncture opening pressure measurements in children. Journal of Neuroophthalmology, 2014;34(3):284-7.
10. Martinez M, Ophir A. Optical coherence tomography as an adjunctive tool for diagnosing papilledema in young patients. Journal of Pediatric Ophthalmology and Strabismus 2011;48(3):174-81.
11. Shah A, Szirth B, Sheng I, et al. Optic disc drusen in a child: diagnosis using noninvasive imaging tools. Optometry and Vision Science. 2013;90(10):269-73.
12. Morris R, Ellerbrock J, Hamp A, et al. Advanced visual field loss secondary to optic nerve head drusen: case report and literature review. Optometry. 2009;80(2):83-100.
13. Carroll S, Gaskin B, Danesh-Meyer H. Giant cell arteritis. Clin Experiment. Ophthalmol. 2006;34(2):159-73.
14. Romero J, Sowka J, Shechtman D. Hemorrhagic complications of optic disc drusen and available treatment options. Optometry. 2008;79(9):496-500.
15. Brown S, Del Monte M. Choroidal neovascular membrane associated with optic nerve head drusen in a child. Am J Ophthalmol. 1996;121(2):215-7.
16. Atta HR. Imaging of the optic nerve with standardized echography. Eye (Lond). 1988;2(Pt 4):358-66.
17. Kulkarni K, Pasol J, Rosa P, Lam B. Differentiating mild papilledema and buried optic nerve head drusen using spectral domain optical coherence tomography. Ophthalmology. 2014;121(4):959-63.
18. Johnson L, Diehl M, Hamm C, et al. Differentiating optic disc edema from optic nerve head drusen on optical coherence tomography. Arch Ophthalmol. 2009;127(1):45-9.
19. Savini G, Bellusci C, Carbonelli M, et al. Detection and quantification of retinal nerve fiber layer thickness in optic disc edema using stratus OCT. Arch Ophthalmol. 2006;124(8):1111-7.
20. Sato T, Mrejen S, Spaide R. Multimodal imaging of optic disc drusen. AM J Ophthalmol. 2013;156(2):275-82.
21. Silverman A, Tatham A, Medeiros F, Weinreb R. Assessment of optic nerve head drusen using enhanced depth imaging and swept source optical coherence tomography. Journal of Neuroophthalmology. 2014;34(2):198-205.
22. Gili P, Flores-Rodriguez P, Yanguela J, et al. Using autofluorescence to detect optic nerve head drusen in children. Journal of AAPOS. 2013;17(6):568-71.
23. Wilkins J, Pomeranz H. Visual manifestations of visible and buried optic disc drusen. Journal of Neuroophthalmology. 2004;24(2):125-9.
24. Hoover D, Robb R, Petersen R. Optic disc drusen in children. Journal of Pediatric Ophthalmology and Strabismus. 1988;25(4):191-5.

