 |
A 40-year-old Hispanic male presented with acute vision loss, redness, pain and photophobia in the left eye. Review of systems revealed that he had been febrile with myalgias and malaise for five days. Prior to presentation, the patient was seen via telemedicine by his general physician, who empirically diagnosed him with influenza and prescribed oseltamivir and acetaminophen, which were not improving his symptoms. He was in otherwise good systemic health with no history of systemic malignancy, human immunodeficiency virus (HIV) infection, intravenous (IV) drug use, prior ocular surgeries or trauma.
Visual acuity (VA) was 20/25 OD and 20/70 OS. Extraocular motilities were full, confrontation visual fields were full and there was no relative afferent pupillary defect. IOP was 12mm Hg OD and 9mm Hg OS by applanation. Anterior and posterior segments were normal OD. Anterior segment examination revealed 1+ diffuse conjunctival hyperemia and 2+ anterior chamber cell and flare OS. Posterior segment imaging is included for review.
 |
Fig. 1. Optos widefield fundus photography of the left eye at presentation. Click image to enlarge. |
 |
|
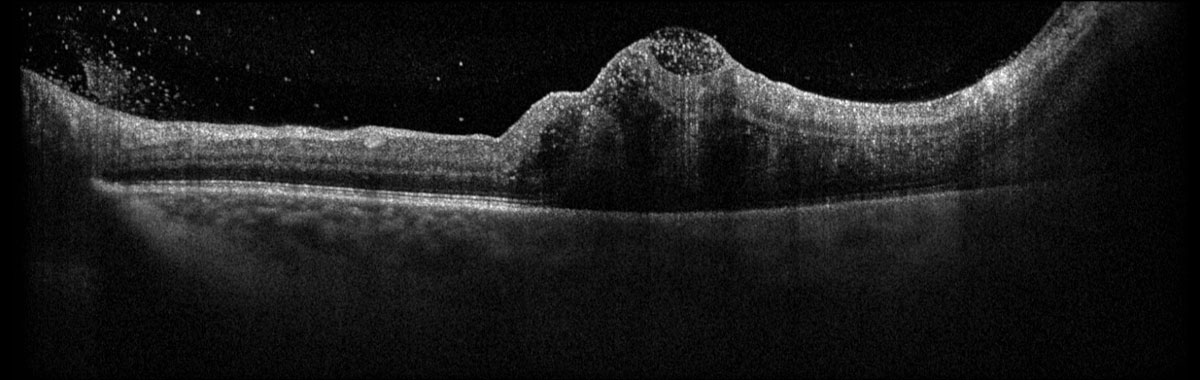
Fig. 2. Heidelberg Spectralis OCT of the left macula at presentation. Click image to enlarge. |
Take the Retina Quiz
1. Which of the following is true of the fundus photo (Figure 1)?
a. There is a solitary mass in the temporal macula.
b. There is extensive retinal vasculitis.
c. There are multifocal Roth spots and intralesional hemorrhages.
d. All of the above.
2. Which is true of the OCT in Figure 2?
a. There are sub-ILM and intraretinal cells.
b. There are vitreous cells.
c. There is a subretinal mass.
d. All of the above.
3. What is the most likely diagnosis?
a. Amelanotic choroidal melanoma.
b. Eales’ disease.
c. Endophthalmitis.
d. Retinoblastoma.
4. Appropriate management includes all of the following except:
a. Hospital admission for blood cultures.
b. Hospital admission for extensive whole-body imaging.
c. Topical antibiotics with one week follow-up in clinic.
d. Vitreous aspiration and intravitreal antibiotics.
5. Which of the following regarding prognosis is true?
a. Visual outcome better than 20/400 is common.
b. Visual outcome of 20/20 is common.
c. Visual outcome of counting fingers or worse is common.
d. This ophthalmic condition carries a 50% risk of mortality.
Diagnosis
Fundus examination OS revealed 1+ vitritis with a large, solitary, dome-shaped, yellow subretinal abscess in the temporal macula with retinal vasculitis, preretinal hypopyon along the inferotemporal arcade, Roth spots and intralesional hemorrhage (Figure 1). OCT depicted vitreous cells, sub-ILM cells, intraretinal cells and partially captured the large subretinal hyperreflective mass in the temporal macula (Figure 2).
Further questioning revealed recent onset chest pain with deep inspiration and abdominal tenderness. The constellation of findings was highly concerning for endogenous endophthalmitis in the absence of exogenous risk factors. Vitreous aspiration was performed, and the patient was administered intravitreal vancomycin (1mg/0.1mL) and ceftazidime (2.25mg/0.1mL) before being transferred to the general hospital for systemic workup to search for an occult infectious source.
The patient underwent extensive full body imaging and blood cultures and a 7.3 x 4.5 x 4.5cm lesion was identified within the right lobe of his liver, consistent with a pyogenic liver abscess (PLA). Interventional radiology proceeded with image-guided percutaneous drainage of the PLA. The PLA and blood cultures both grew Klebsiella pneumoniae (K. pneumoniae).
Discussion
Endophthalmitis is a rare but devastating ocular infection that can involve the entire globe, and its source can be exogenous or endogenous.1 Exogenous endophthalmitis is far more common (more than 90% of endophthalmitis cases), and typical risk factors include recent ocular surgery, open-globe injury and recent intravitreal injection.1,2 Endogenous endophthalmitis occurs via hematogenous dissemination of a systemic infectious source.1-3 Typical risk factors include diabetes mellitus, immunosuppression/compromise (e.g., chemotherapy or HIV infection), indwelling catheters and IV drug use.1,4,5
Klebsiella species are gram-negative, encapsulated anaerobic bacteria commonly found in nasopharyngeal and gastrointestinal flora.2,6 K. pneumonia is capable of producing pneumonia, urinary tract infections, PLA, meningitis and endophthalmitis (known as KPEE).7 The presence of K. pneumoniae PLA portends a poorer visual prognosis, as well as up to 10% risk of mortality.5,8
The infectious source for KPEE is most frequently found in the hepatobiliary tract, with K. pneumoniae PLA present in greater than 75% of patients, followed by pneumonia and urinary tract infections.9 Furthermore, nearly one in 12 patients with K. pneumoniae PLA will develop KPEE, and the risk increases four-fold for K. pneumoniae PLA measuring greater than 5cm.10 While KPEE is most prevalent in Asian countries and in patients of Asian descent, there are increasing reports of cases in the United States and in non-Asian patients.11
 |
|
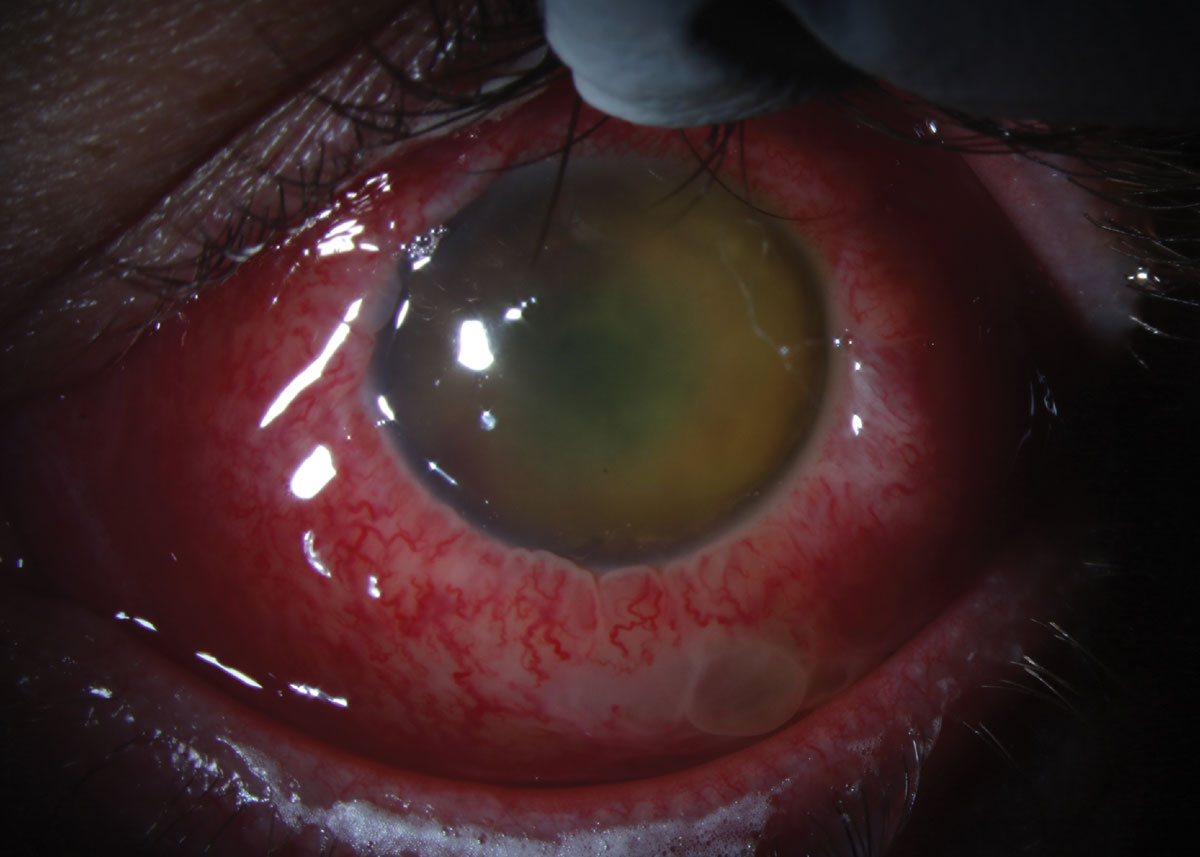
Fig. 3. Slit lamp photograph of the left eye at follow-up after 13 days. Click image to enlarge. |
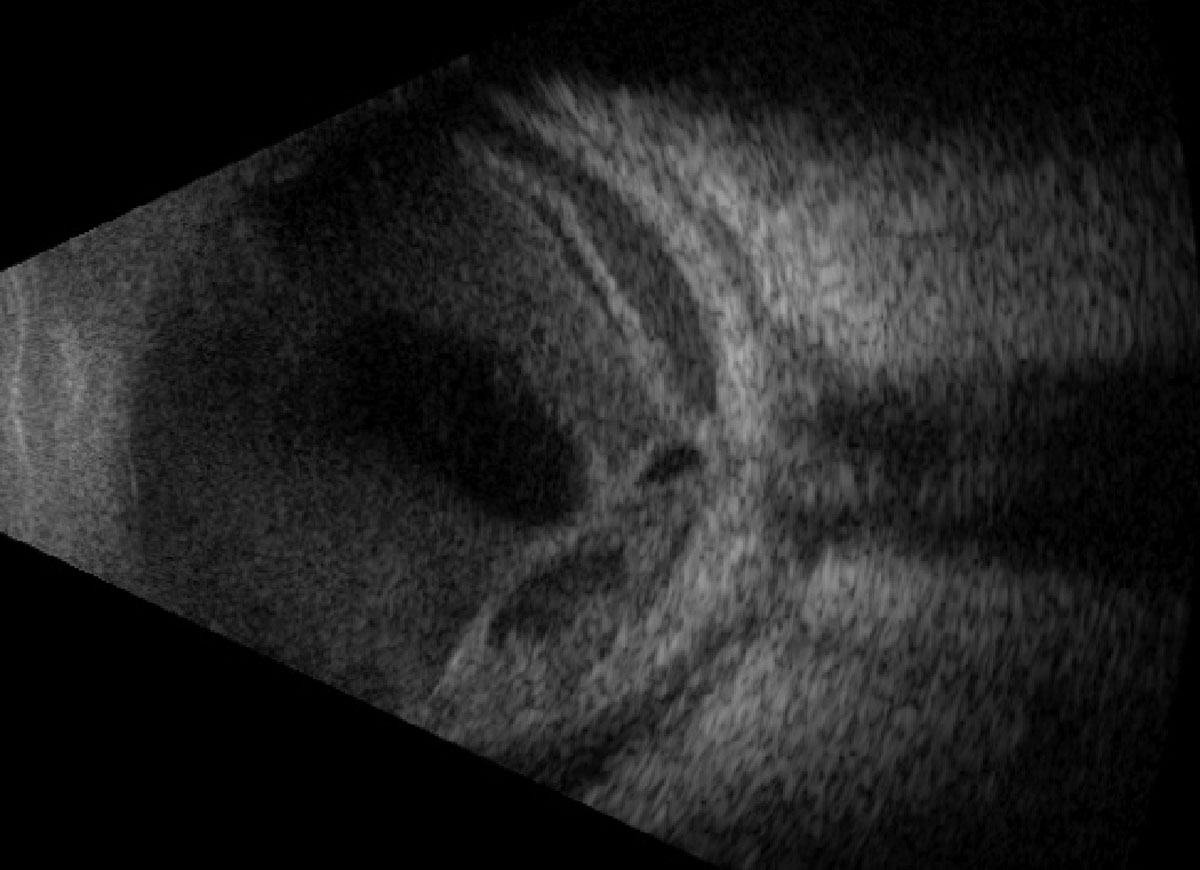 |
Fig. 4. Vertical axial B-scan over the posterior pole at follow-up after 13 days. Click image to enlarge. |
Presently, capsular phenotyping of K. pneumonia is available but not routinely performed in the US.11,12 Capsular serotypes K1 and K2 are most virulent due to increased resistance/decreased susceptibility to neutrophil phagocytosis; as such, they are deemed hypervirulent strains, and the association with K. pneumonia hypervirulent K1 and K2 serotype bacteremia and KPEE is well-established.5,11,12
The most frequent symptoms seen in patients of a recent study with subretinal abscess secondary to endogenous endophthalmitis were vision loss (70%), pain (65%) and redness (35%).3 Anterior chamber reaction, conjunctival hyperemia and hypopyon were seen in less than 75% of patients presenting with subretinal abscess, emphasizing the need for dilated fundus examination of every patient presenting with intraocular anterior segment inflammation.3
There is a broad differential diagnosis that includes other infectious (tuberculosis, viral, toxoplasmosis, syphilis) and inflammatory (sarcoidosis) entities, as well as neoplastic processes such as intraocular lymphoma, leukemic infiltration, retinoblastoma and primary and metastatic tumors to the uvea or vitreous.1 When the clinical presentation and/or review of systems is suggestive of endogenous endophthalmitis or septicemia in the absence of known systemic illness, emergent systemic workup and infectious disease consultation is necessary to identify the source.1 Some reports suggest final VA in patients with KPEE is correlated directly with initial VA; however, the vast majority of patients with KPEE develop final VA of counting fingers or worse.2,10,13
Treatment
Our patient received intravitreal vancomycin and ceftazidime prior to and during hospital admission, as well as IV antibiotics. Despite prompt recognition of the disease and detection of the systemic source, his vision rapidly declined to light perception within days. Systemically, the bacteremia was treated to resolution with systemic antimicrobial therapy. Upon discharge 13 days later, the patient returned with a significant fibrinous anterior chamber reaction and dense membranous vitritis with total retinal detachment (Figures 3 & 4). Observation was recommended, and comfort was achieved with topical difluprednate and atropine.
Though endophthalmitis is rare, optometrists must maintain a high index of suspicion for both exogenous and endogenous forms, as patients may initially present with acute or subacute vision loss. Clinical presentation can be variable, and the implication of delayed recognition carries risk of severe vision loss and mortality.
Finally, optometrists taking call at general hospitals for inpatient consultations must recognize the important association between K. pneumoniae septicemia, PLA and KPEE, and careful dilated fundus examination must be performed on all patients to rule out intraocular involvement.
Retina Quiz Answers
1: d, 2: d, 3: c, 4: c, 5: c
 |
Drs. Aboumourad and Leone currently practice at Bascom Palmer Eye Institute in Miami. Neither has any financial disclosures.
Dr. Dunbar is the director of optometric services and optometry residency supervisor at the Bascom Palmer Eye Institute at the University of Miami. He is a founding member of the Optometric Glaucoma Society and the Optometric Retina Society. Dr. Dunbar is a consultant for Carl Zeiss Meditec, Allergan, Regeneron and Genentech.
1. Ryan SJ, Davis JL, Flynn HW, et al. Retina, Fifth ed. London; New York: Saunders/Elsevier, 2013. 2. Arjamilah MN, Aiman-Mardhiyyah MY, Shatriah I, et al. Bilateral Endogenous Klebsiella pneumoniae Endophthalmitis in Culture-Negative Liver Abscess Requiring Evisceration: A Case Report and Review of Literature. Cureus 2023;15(3):e36965. 3. Gallo B, Testi I, Pavesio C. Subretinal abscess: causative pathogens, clinical features and management. J Ophthalmic Inflamm Infect. 2022;12(1):40. 4. Schiedler V, Scott IU, Flynn HW, Jr., et al. Culture-proven endogenous endophthalmitis: clinical features and visual acuity outcomes. Am J Ophthalmol. 2004;137(4):725-31. 5. Sridhar J, Flynn HW Jr., Kuriyan AE, et al. Endophthalmitis caused by Klebsiella species. Retina 2014;34(9):1875-81. 6. Chang FY, Chou MY. Comparison of pyogenic liver abscesses caused by Klebsiella pneumoniae and non-K. pneumoniae pathogens. J Formos Med Assoc. 1995;94(5):232-7. 7. Correia C, Lopes S, Mendes S, et al. Endogenous endophthalmitis and liver abscess: a metastatic infection or a coincidence? GE Port J Gastroenterol. 2022;29(6):426-31. 8. Yang G, Huang X, Jiang S, Xu Z. Endogenous endophthalmitis caused by Klebsiella pneumoniae: a ten-year retrospective study in Western China. Ophthalmic Res. 2020;63(5):507-16. 9. Chen KJ, Chen YP, Chen YH, et al. Infection sources and Klebsiella pneumoniae antibiotic susceptibilities in endogenous Klebsiella endophthalmitis. Antibiotics (Basel). 2022;11(7). 10. Kim E, Byon I, Lee JJ, et al. Endogenous endophthalmitis from a Klebsiella pneumoniae liver abscess: the incidence, risk factors and utility of imaging. Am J Ophthalmol. 2023;252:69-76. 11. Kashani AH, Eliott D. The emergence of Klebsiella pneumoniae endogenous endophthalmitis in the USA: basic and clinical advances. J Ophthalmic Inflamm Infect. 2013;3(1):28. 12. Lin JC, Siu LK, Fung CP, et al. Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor glycemic control. J Clin Endocrinol Metab. 2006;91(8):3084-7. 13. Chen YJ, Kuo HK, Wu PC, et al. A 10-year comparison of endogenous endophthalmitis outcomes: an east Asian experience with Klebsiella pneumoniae infection. Retina 2004;24(3):383-90. |

