Spotlighting OSDMedication, infection, genetics, poor hygiene—these are only a few factors that contribute to ocular surface disease. This month in Review of Optometry, experts weigh in on the risk factors, diagnosis, treatment and management of numerous types of OSD. Check out the other articles featured in the November issue:
|
Adenoviral conjunctivitis (Ad-Cs) is a prevalent, highly contagious condition that has considerable morbidity and economic impact. Despite this, there are no FDA-approved treatments for Ad-Cs. The off-label use of 5% povidone-iodine (PVP-I)—a broad-spectrum antiseptic with an excellent safety profile—has been adopted by some clinicians to treat patients with Ad-Cs. But how effective is this intervention really?
A pilot clinical trial—the Reducing Adenoviral Patient Infected Days (RAPID) study, completed in 2018—assessed if a single, in-office treatment with 5% PVP-I reduces virus-positive days more rapidly than artificial tears alone (n=28). This decade-long research project has provided numerous insights, including some you may not have suspected regarding the diagnosis and management of this challenging, terribly uncomfortable and potentially sight-threatening infection.
In this article, I’ll discuss some of the main findings of the RAPID study and debunk a few myths about managing this infectious disease. But first, let’s review what exactly a diagnosis of Ad-Cs means for you and your patient and the potential effects it can have on vision and the eye.
What is Ad-Cs?
Often referred to as “pink eye,”Ad-Cs is one of the most common eye infections worldwide. The proportion of infectious conjunctivitis cases that are due to viral infections may be as high as 80%, and 65% to 90% of viral infections are thought to be a result of adenoviruses.1 Patients with acute conjunctivitis typically present to a primary care provider, comprising as much as 2% of a general practitioner’s practice.2 A population-based incidence study of eye-related emergency department visits reported two million visits per year, with 28% of these visits being related to a diagnosis of acute or other types of conjunctivitis.3
The following are members of the RAPID study steering committee and contributed to the research presented in this article:
|
Ad-Cs is more contagious than other forms of conjunctivitis partly due to the virus’s ability to remain infectious in a desiccated state for weeks at room temperature.4 Adenoviruses have no outer lipid bilayer and are highly resistant to standard disinfectants, including 70% isopropyl alcohol and 3% hydrogen peroxide.5 The virus is transmitted directly through droplets or smears of infected bodily fluids, primarily tears or respiratory secretions and by fomites on towels, doorknobs, pens, counters, instruments, eye drops or eyeglasses.6
In a small study by Azar et al., the hands of nearly 50% of patients with Ad-Cs presenting for care were culture-positive, compared to 0% of patients who didn’t have the infection.6 Secondary transmission of Ad-Cs to members of the same household is estimated to occur at a rate of 20%.7 Outbreaks frequently occur in schools, military units, nursing homes, workplaces and community and healthcare facilities.7-13
Due to the highly transmissible nature of the condition, its economic impact is a significant concern. An estimated $670 million is spent annually on the medical management of Ad-Cs.14 Not only do companies likely suffer from the absence of multiple employees at once, but infected workers may also be forced to take a leave without pay until they are no longer symptomatic (which could take up to four weeks). One large study noted that infected workers typically receive a one- to two-week furlough, potentially causing a loss of 25% to 50% in monthly wages (of course, paid time off may vary by employer).15
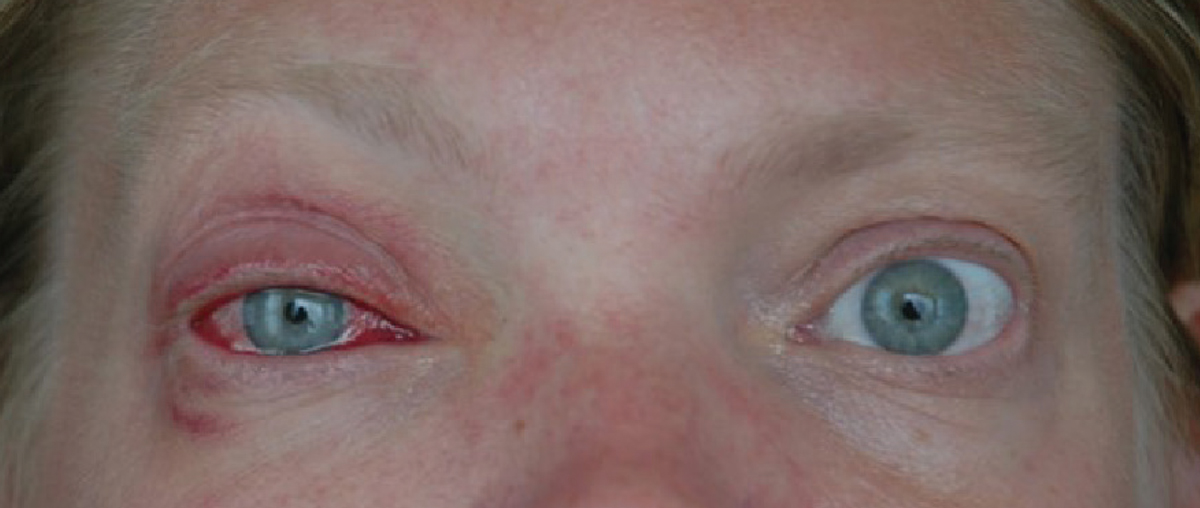 |
| Fig. 1. A patient with unilateral adenoviral conjunctivitis. Click image to enlarge. |
Ocular Effects
Ad-Cs is highly symptomatic, causing discomfort, tearing, lid swelling, photophobia and decreased vision. Ocular signs include bulbar conjunctival redness, chemosis, follicular reaction and subconjunctival hemorrhage. Ad-Cs is also often associated with a palpable preauricular node. Ocular sequelae include the formation of pseudomembranes and subepithelial corneal infiltrates. Approximately 15% to 48% of Ad-Cs patients develop subepithelial corneal infiltrates, which can cause permanent corneal stromal scarring and may lead to vision loss.16-19 Clinicians typically use symptoms and clinical signs to diagnose Ad-Cs, but this is complicated by their variability in presence and severity among infected individuals.20
The incubation period for adenoviral ocular infections is two to 14 days prior to symptom onset and symptoms typically persist for seven to 28 days.21,22 The period of contagion lasts approximately three weeks. Studies report that most eyes are culture-negative by 13 days after the onset of symptoms.13,23 The duration and severity of symptoms and complications differ among the more than 20 adenovirus serotypes associated with ocular infections; however, serotypes 8, 19, 37 and 53 are known to have the greatest epidemic potential.24
Confirming the Diagnosis
An accurate diagnosis of Ad-Cs is essential for timely and appropriate management to reduce transmission, duration, severity and complications of Ad-Cs. However, a recent meta-analysis of 77 publications concluded that clinicians cannot reliably differentiate between bacterial and viral conjunctivitis based on clinical signs and symptoms.25 In a systematic review of more than 6,800 publications, a study by Rietveld et al. was unable to find evidence of the clinical signs or symptoms that were useful for the differential diagnosis of bacterial from viral conjunctivitis.26
The percentage of clinically diagnosed Ad-Cs cases for which adenoviral etiology is confirmed by polymerase chain reaction (PCR) testing is variable, with estimates ranging from 8% to 82%, highlighting the challenge of accurate diagnosis.14,27-31 While PCR testing is the gold standard for adenoviral detection, the technique is costly and time-consuming, usually requiring off-site testing that prevents immediate diagnosis. In 2006, the FDA approved a CLIA-waived, point-of-care test for acute conjunctivitis called the QuickVue adenoviral conjunctivitis test (previously known as AdenoPlus; Quidel Corp.). In 10 minutes, this point-of-care test provides a binary “yes” or “no” result for the presence of adenovirus antigen down to a lower limit of 40 to 50 virions. Sensitivity has been reported to range from 40% to 93% and specificity from 81% to 98%.33-36
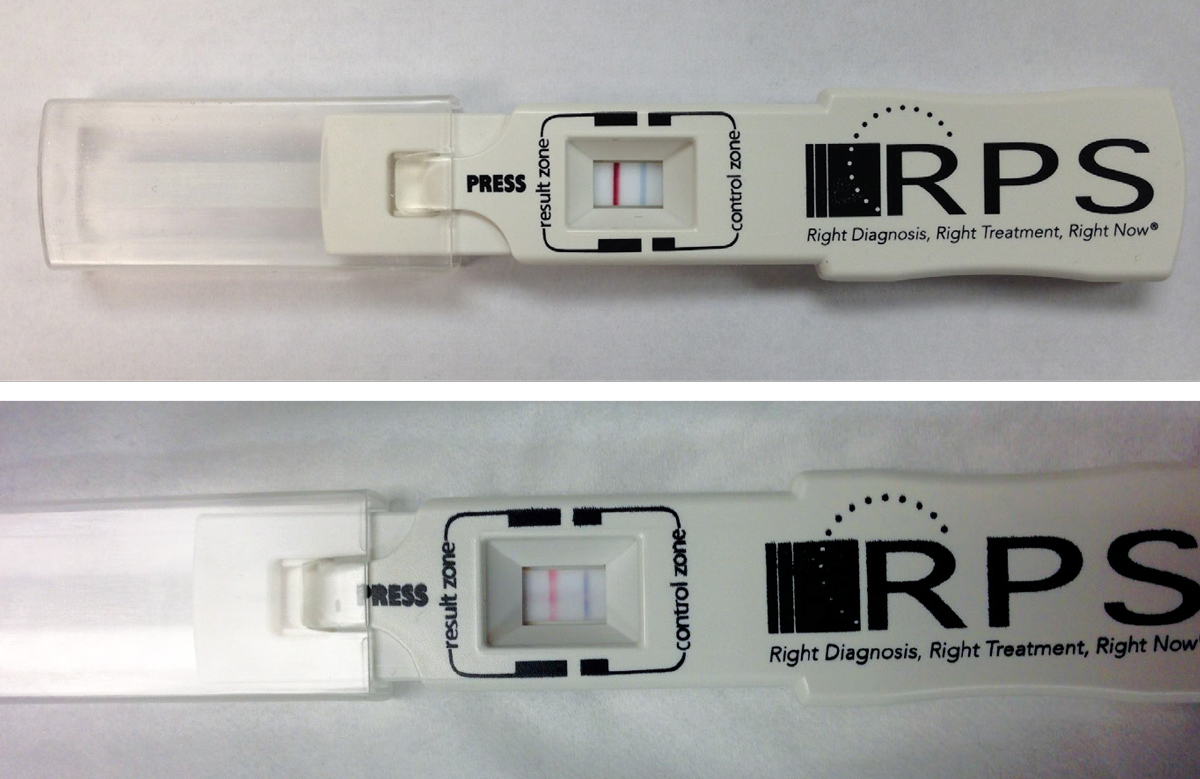 |
| Fig. 2. Shown here are two positive point-of-care tests using the RPS Adeno Detector (Rapid Pathogen Screening). The blue line is the control; presence of the red line indicates a positive test result for Ad-Cs. The top image is prior to treatment with PVP-I; the bottom image is 24 hours post-treatment. Click image to enlarge. |
A survey of eyecare providers at a national meeting found that only 10% of the 340 attendees reported use of this point-of-care test. This highlights the current lack of a widely accepted, inexpensive, in-office test that can accurately determine the presence of Ad-Cs.37
Treatment Options
Not only is it challenging to make an accurate diagnosis, the actual management of this form of conjunctivitis presents its own unique hurdles, as there aren’t any FDA-approved treatments for Ad-Cs. Antibiotics are commonly prescribed for conjunctivitis regardless of causative agent.15 Analysis of claims data in a large managed care network found that nearly 60% of the patients diagnosed with acute conjunctivitis filled an antibiotic prescription, and 20% were prescribed an antibiotic-corticosteroid product.38 Unnecessary or inappropriate use of antibiotics is partly attributable to the difficulty in discriminating between viral and bacterial conjunctivitis. Both the American Optometric Association clinical practice guidelines and American Academy of Ophthalmology preferred practice guidelines recommend supportive care consisting of artificial tears, topical antihistamines and cold compresses.39,40
Several antiviral agents have been tested but the adenoviral structural characteristics have proven too hardy for these medications. Because of the significant morbidity and transmissibility associated with this form of conjunctivitis, there are ongoing drug development efforts to find an effective FDA-approved agent.
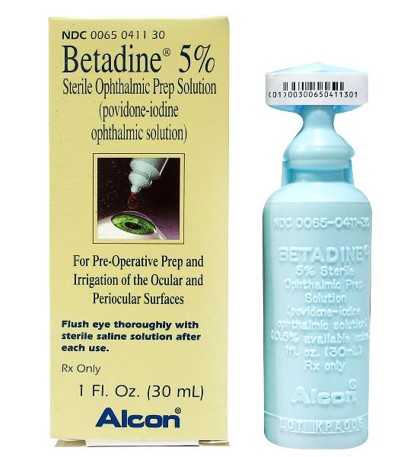 |
|
Fig. 3. Ophthalmic formulation of PVP-I. Click image to enlarge. |
Until such a drug reaches market, several medications are being used off-label, including a one-time application of PVP-I. Iodine has been used as an antiseptic since 1839. An ophthalmic formulation of povidone-iodine is available at a concentration of 5% (Betadine 5%, Alcon) while the concentration u for dermatologic applications is 10% (Figure 3).
Ophthalmic PVP-I is FDA-approved for “the prepping of the periocular region (lids, brow and cheek) and irrigation of the ocular surface (cornea, conjunctiva and palpebral fornices).” PVP-I has been shown to be effective against bacteria, including chlamydia, fungi and protozoa, as well as viruses, including adeno, herpes simplex and enteroviruses, without significant corneal or other ocular toxicity. Compared to other antiseptic agents, it has a wider virucidal spectrum.9
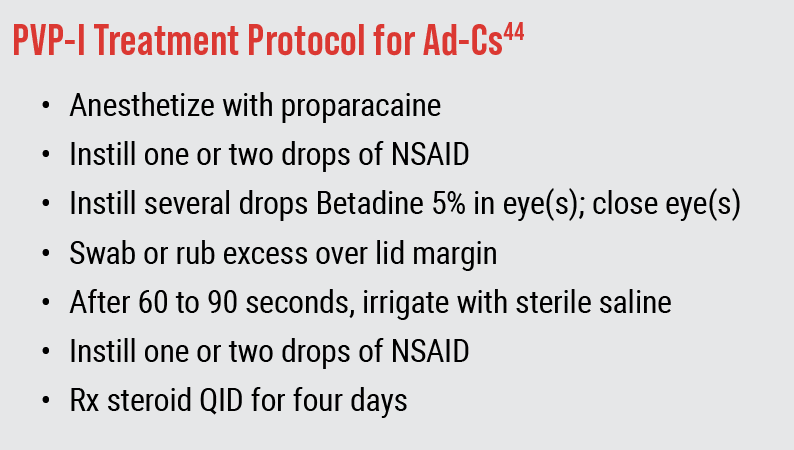
More than two decades ago, a study recommended that 5% PVP-I was a “good treatment for such external ocular infections as Ad-Cs.”41 This “off-label” use of 5% PVP-I as a treatment option for Ad-Cs has continued to gain credence, with its promotion in influential editorials and reviews that have wide distribution within the eyecare community.42,43 In their annual drug guide, Randall Thomas, OD, MPH, and Ron Melton, OD, recommend a protocol for using PVP-I based on their clinical experience and research (Figure 4).44 This same regimen is recommended in other literature for treating cases of suspected COVID-19-related viral conjunctivitis, as PVP-I is effective against this virus as well.45
 |
|
Fig. 4. Protocol of Drs. Melton and Thomas. In an effort to minimize confounding factors, RAPID study did not use topical NSAID or steroid, used a contact time of two minutes and prescribed preservative-free artificial tears four times a day. |
Despite the lack of well-designed clinical studies, a 2013 survey of more than 600 eyecare providers found that one-third used off-label 5% PVP-I as part of their management of Ad-Cs (Figure 5).37 Only a few clinical trials have investigated the efficacy of PVP-I alone for Ad-Cs; more have assessed PVP-I in combination with dexamethasone. These studies suggest that PVP-I alone can effectively reduce viral titers, symptom severity and/or duration. Larger, double-masked, placebo-controlled randomized trials of fixed-combination PVP-I and dexamethasone have shown efficacy in reducing the severity and duration of Ad-Cs.
Earlier this year, a Cochrane review by Liu et al. found that the current available evidence is insufficient to determine whether any of the evaluated interventions including PVP-I confer an advantage over steroids or artificial tears with respect to virus eradication (epidemic keratoconjunctivitis) or its spread to initially uninvolved fellow eyes. However, data from the RAPID study concludes that this pharmaceutical in particular may have more merit as a treatment for Ad-Cs than previously shown.
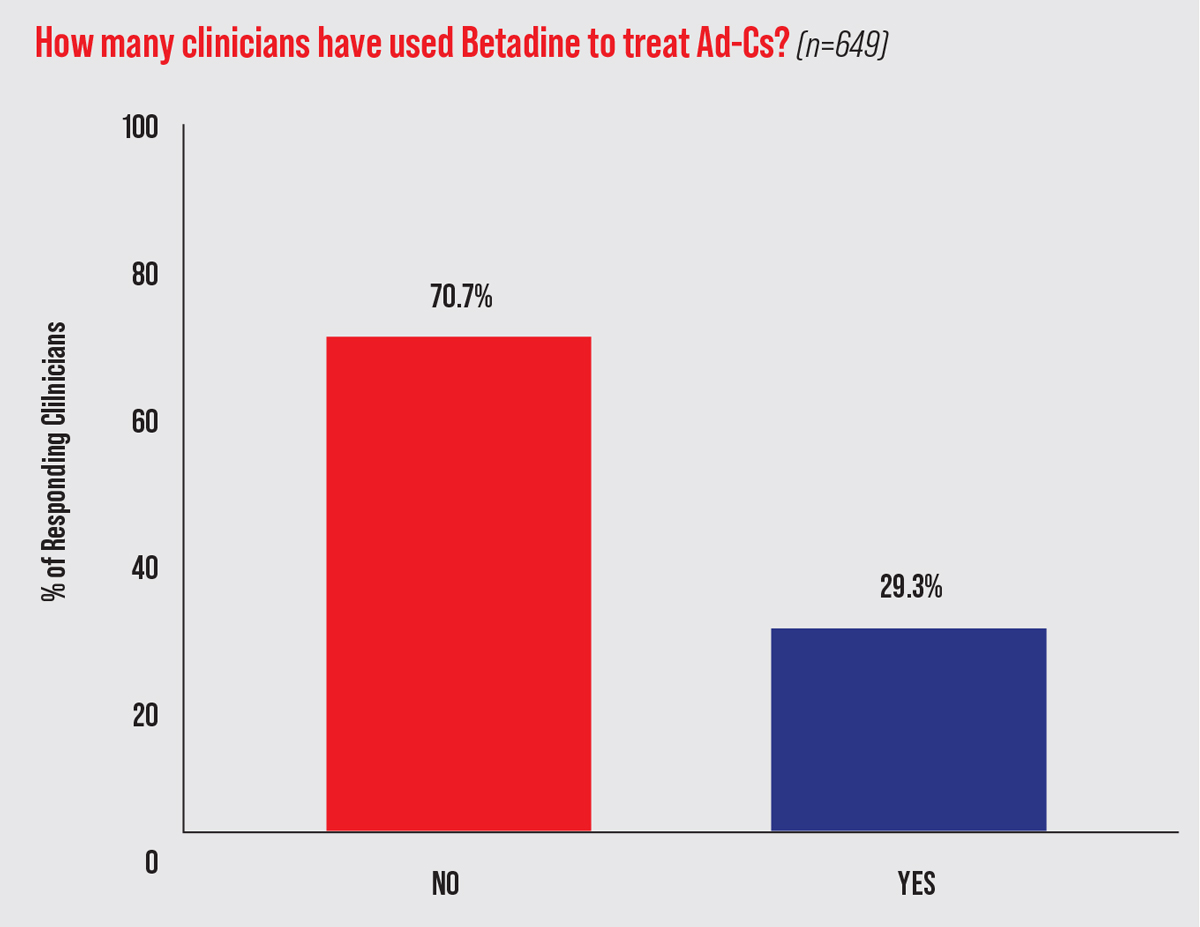 |
| Fig. 5. PVP-I usage by optometrists and ophthalmologists for presumed Ad-Cs. This data comes from the survey responses of 649 clinicians from seven eyecare meetings in 2013.37 Click image to enlarge. |
The RAPID Study
In this pilot clinical trial funded by the National Institutes of Health and conducted by a team of colleagues and I, patients who presented with presumed Ad-Cs were screened at nine US clinics. They were eligible to be enrolled if they were at least 18 years old, had symptoms consistent with Ad-Cs for four days or less and had a positive point-of-care (AdenoPlus) test.
Patients were randomized to receive a single administration in-office on the day of enrollment of 5% PVP-I or artificial tears in one eye. To avoid confounding factors, no topical steroids nor NSAIDs were used. Patients were to use preservative-free artificial tears four times daily while symptomatic. Serial quantitative polymerase chain reaction (qPCR) analyses were performed to determine the impact of treatment on viral titers over a period of three weeks. Clinical signs and symptoms were assessed over the three-week period (days one to two, four, seven, 14 and 21) comparing the effect of PVP-I and artificial tears administered a single time in-office. The primary outcome was percent reduction from peak viral load. Secondary outcomes were improvement in clinical signs and symptoms. Some of the study’s conclusions are discussed in the next section.
Ad-Cs: Fact or Fiction?
Let’s dissect a few common myths—or truths—about Ad-Cs using findings from the RAPID study and other current literature. Test your clinical understanding and see how many of the following factual or fictitious statements about the condition may come as a surprise to you.
1. The majority of conjunctivitis is caused by adenovirus. FICTION!
In our study, we recruited patients suspected of having Ad-Cs based on a set of commonly accepted, predetermined clinical signs and symptoms. Of the 212 screened, only 56 had a positive point-of-care test (26.4%). Of those 56, only 28 had the presence of adenoviral titers based on qPCR. Therefore, of the 212 presenting with presumed Ad-Cs, in fact only 13.2% were true positives (28 out of 212).
The literature reports a wide range of prevalence, but one large study at Johns Hopkins University with 1,520 individuals with suspected Ad-Cs demonstrated a surprisingly low percentage—only 8.6%—were positive for adenovirus through PCR testing.46
Studies by Kam and Holtz report PCR-confirmed diagnoses in patients presenting with presumed Ad-Cs are 39.4% and 21.7%, respectively.46 These results indicate that many patients suspected to have Ad-Cs based on clinical signs and symptoms are likely unnecessarily quarantined. The question of what causes the more than 80% of presumed Ad-Cs cases remains yet to be answered. One possible cause is some other viral infection, but further research is needed.
2. Betadine is intolerable and therefore not a good option for patients. FICTION!
While in vitro and animal models of PVP-I have demonstrated corneal epithelial cell toxicity, several study limitations challenge the validity of these findings. For example, the PVP-I concentrations used were much higher than what is used clinically, ophthalmic formulations were not used and, in two studies particularly, PVP-I was injected directly into the anterior chamber.14 A small study of 10 healthy normal subjects reported corneal epithelial changes after application of 5% PVP-I that resolved within 24 hours. Another study, by Saedon et al., reported increased dry eye symptoms and corneal epithelial staining in eyes that received multiple intravitreal injections preceded by PVP-I; however, the eyes were compared to fellow eyes receiving neither intravitreal injections nor PVP-I.47-49
According to Marks, the safety of PVP-I has been demonstrated by the lack of reports of significant toxicity or poor tolerability in animals and humans.50 In the RAPID study, corneal staining increased immediately post-administration of PVP-I but returned to baseline levels within hours. There was no change in visual acuity between baseline and day one. It was reassuring to note that there was no change in participant-rated overall discomfort immediately post-administration of PVP-I or on day one compared to baseline. These results suggest ophthalmic 5% PVP-I used as a one-time treatment is safe and well tolerated by patients with Ad-Cs. Assessment of the effectiveness of masking provides additional implicit evidence of PVP-I tolerability. During informed consent, the study patients were educated regarding the potential stinging of PVP-I.
All treated patients were asked immediately following in-office treatment as to whether they believed they received PVP-I, artificial tears or were not sure. Thirty-four percent of participants treated with PVP-I guessed incorrectly or were unsure which treatment they received. Presumably if PVP-I caused significant discomfort upon instillation, participants would have had a much higher correct-guess rate.51
3. QuickVue adenoviral conjunctivitis test has no clinical utility. FICTION!
This point-of-care test had relatively low-positive predictive power for Ad-Cs, as only 50% of the point-of-care test-positive patients had viral titers on qPCR testing.36 This is consistent with some of the previous reports.
Considering this data in isolation, one would presume that this point-of-care test is no better than random chance. However, where the value of this test lies is in its excellent negative predictive power; 98% of those that were negative on point-of-care testing did not have presence of viral load on qPCR testing. This finding has clinical implications, as negative test results can indicate non-adenoviral etiology to the clinician, greatly altering management and quarantine recommendations. Confirmation that the conjunctivitis is not adenoviral has significant economic impact by avoiding the need to take time off work.
4. Betadine prevents the formation of SEIs and pseudomembranes. FICTION!
It was hypothesized that 5% PVP-I would reduce the incidence of ocular sequelae of Ad-Cs consistent with what was anecdotally observed prior to this study. However, the incidence of corneal infiltrates and pseudomembranes was higher in the 5% PVP-I group compared to those receiving artificial tears, although not statistically significant. Fourteen of 25 participants (56%) who were qPCR-positive for adenovirus developed either a subepithelial infiltrate (seven in the PVP-I group and four in the artificial tear group) or a pseudomembrane (three in the PVP-I group and one in the artificial tear group). One participant had both complications. These findings were not expected, and the small sample size makes interpretation challenging. Still, these results show that a one-time in-office application of 5% PVP-I clearly did not prevent ocular sequelae of Ad-Cs.
The overall incidence (both treatment groups) of pseudomembranes in our study was 16%, which is comparable to the 24% reported by Butt et al. in a retrospective observational study.52 The incidence of infiltrates was 56% in this study and is consistent with the Lee et al. prospective study of 500 patients in four countries, which reported an incidence of 59% over 18 days of follow-up.32 A retrospective study of 110 patients in the United States reported an incidence of infiltrates of 49%.52 Serotyping was not performed, though that information may have been useful in identifying which serotypes were associated with development of infiltrates or pseudomembranes.
5. Resolution of signs and symptoms of Ad-Cs indicate viral clearance. FICTION!
Resolution of Ad-Cs is typically determined either based on the “typical” time course of the disease or by monitoring for clearing of key signs and symptoms. Because of the nature of this study, we were able to perform qPCR testing from each visit (which is impractical in a clinical setting) analyzing the relationship of viral titers to clinical signs and symptoms. Not surprisingly, higher baseline viral titers resulted in more severe symptoms and signs and these cases took longer for viral clearance. It was found that most signs and symptoms persisted after viral clearance.
Of all the signs and symptoms typically used clinically to aid in the diagnosis of Ad-Cs, it appears that the disappearance of serous discharge most closely correlates with the viral clearance. Using the resolution of signs and symptoms (other than serous discharge) to determine when patients are no longer contagious likely results in delayed release from quarantine.
In another analysis, researchers found that viral titers are detected by qPCR after the point-of-care testing yields negative results. So, even though the point-of-care test is more accessible, cheaper and provides nearly immediate results (10 minutes), patients with Ad-Cs may still have detectable virus more than 14 days after initial presentation. This highlights the need for development of a rapid, inexpensive method for assessing viral titers.
6. Lymph nodes are present in the majority of Ad-Cs cases. FICTION!
Classic training has taught clinicians to expect most if not all patients presenting with Ad-Cs will have a preauricular node. Our study found 46% of those with qPCR confirmed Ad-Cs to have a preauricular node at initial presentation. Nodal involvement increased to 56% if other palpated nodes (e.g., retroauricular, submandibular) were included. This still indicates that a significant number of Ad-Cs cases do not present with the classic preauricular node, so its absence doesn’t rule the disease out as one of several potential diagnoses.
7. Eyelid swelling is more useful than follicles in diagnosing Ad-Cs. FACT!
Using multivariate modeling, clinician-graded conjunctival redness, participant-reported eyelid edema and overall discomfort were the three clinical findings that were highly predictive in identifying individuals with PCR-confirmed Ad-Cs.53 Combining the scores of these three clinical signs/symptoms with the results of an adenoviral point-of-care test further improved the predictive accuracy of correctly identifying Ad-Cs. This may appear confusing based on the apparent inaccuracy of the point-of-care test; however, when combined with key clinical signs and symptoms, the predictive ability of the presence of Ad-Cs improved significantly. Improving diagnostic accuracy for Ad-Cs by incorporating both point-of-care tests and clinical evaluation of key signs and symptoms could prevent unnecessary work furloughs and facilitate earlier management decisions for clinicians.
 |
|
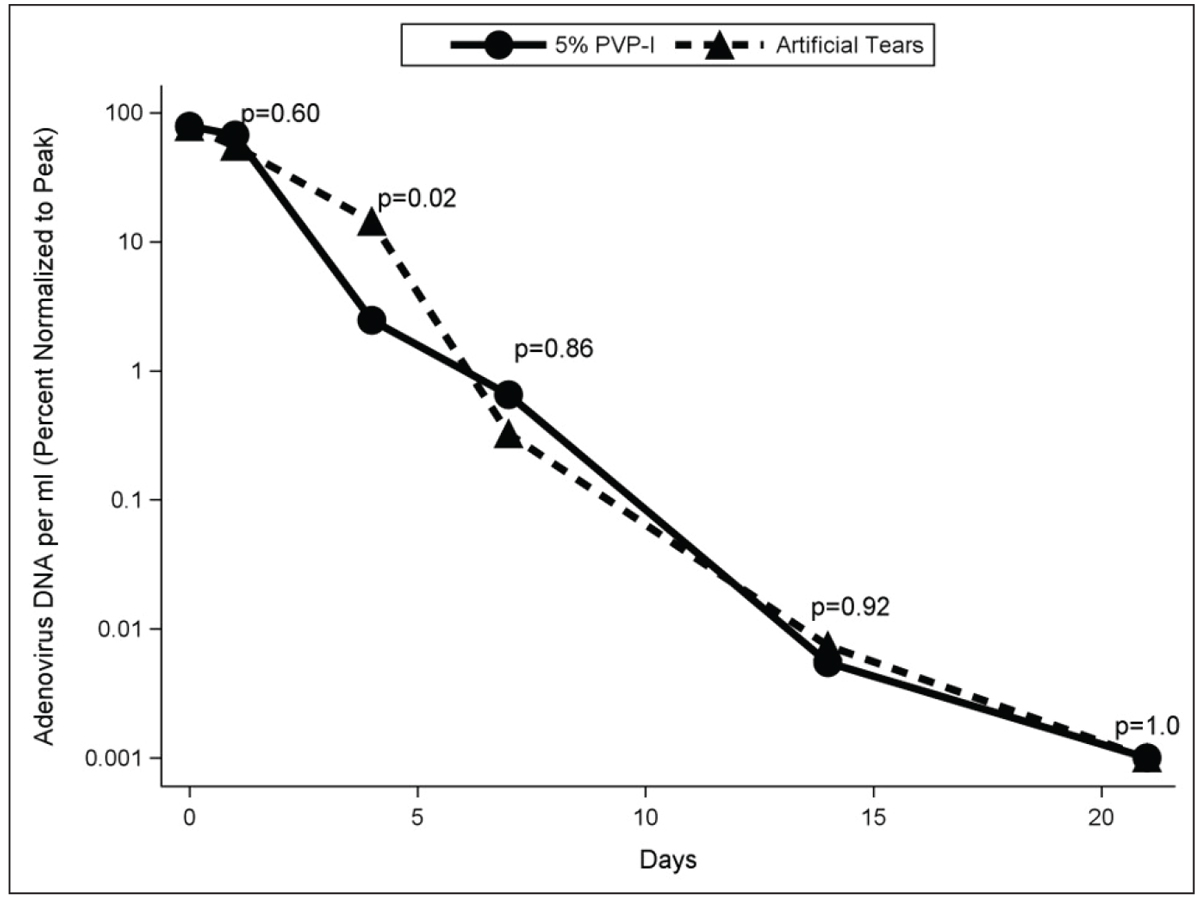
Fig. 6. Percent of peak viral load for 5% PVP-I and artificial tears groups at days one to two, four, seven, 14 and 21 among participants qPCR + for adenovirus. Click image to enlarge. |
8. PVP-I has no value in management of Ad-Cs. YOU BE THE JUDGE!
Four days after treatment in the RAPID study, viral titers in the 5% PVP-I and artificial tear groups were 2.5% ± 2.7% and 14.4% ± 10.5% of peak, respectively (Figure 6).20 Severity of patient-reported symptoms as well as clinician-graded signs were lower in the 5% PVP-I group than artificial tear group on day four. After day four, viral titers and severity of signs and symptoms decreased markedly in both groups and no differences between groups were detected.
Bottom line is that PVP-I, while not perhaps as robust as previously thought, resulted in patients feeling and looking better at day four, which is consistent with reduced viral load. After that time point, the resolution parallels the natural course. Based on this observation, it appears that offering PVP-I to patients presenting early in the course of the disease is warranted.
 |
|
Epidemic keratoconjunctivitis in a 30-year-old woman. Photo: Joseph Sowka, OD. Click image to enlarge. |
Takeaways
These two words sum up our conclusions about the use of PVP-I: educate and offer. Until we have a rapid PCR test available and an FDA-approved medication for Ad-Cs, we can use the knowledge gained from current literature such as the RAPID study to help guide management of infected patients, who should also be informed of their therapeutic options. In addition to PVP-I, supportive care such as artificial tears and cool compresses is still warranted. Other medications such as topical steroids, NSAIDs and topical ganciclovir continue to be used off-label in the management of Ad-Cs.
The next time a patient presents to your office with presumed Ad-Cs, consider using the point-of-care test. If negative, you be quite confident (with 98% certainty) that it’s not Ad-Cs. If positive, there is a greater chance that it is Ad-Cs. Consider offering PVP-I to your patients with adenoviral keratoconjunctivitis to help them feel better sooner, keeping in mind that infiltrates may still occur.
Dr. Than is a staff optometrist at the Carl Vinson VA Medical Center in Dublin, Georgia. She was formerly a professor at the University of Alabama at Birmingham School of Optometry. Dr. Than publishes in several optometric journals and lectures nationally on various subjects including therapeutics, dermatology and ophthalmic lasers. She has received numerous optometry and teaching awards and is also a Fellow of the American Academy of Optometry. Dr. Than has no financial interests to disclose.
1. Kuo IC. Adenoviral keratoconjunctivitis: Diagnosis, management, and prevention. Curr Ophthalmol Rep. 2019(7):118-27. 2. Abel R, Abel AD. Use of povidone-iodine in the treatment of presumptive adenoviral conjunctivitis. Ann Ophthalmol Glaucom. 1998;30(6):341-3. 3. Channa R, Zafar SN, Canner JK, et al. Epidemiology of eye-related emergency department visits. JAMA Ophthalmol. 2016;134:312-19. 4. Chaberny IE, Schnitzler P, Geiss HK, Wendt C. An outbreak of epidemic keratoconjunctivitis in a pediatric unit due to adenovirus type 8. Infect Control Hosp Epidemiol. 2003;24(7):514-9. 5. Gordon YJ, Gordon RY, Romanowski E, Araullo-Cruz TP. Prolonged recovery of desiccated adenoviral serotypes 5, 8, and 19 from plastic and metal surfaces in vitro. Ophthalmol. 1993;100(12):1835-40. 6. Azar MJ, Dhaliwal DK, Bower KS, Kowalski RP, Gordon YJ. Possible consequences of shaking hands with your patients with epidemic keratoconjunctivitis. Am J Ophthalmol. 1996;121(6):711-2. 7. Colon LE. Keratoconjunctivitis due to adenovirus type 8: Report on a large outbreak. Ann Ophthalmol. 1991;23(2):63-5. 8. Jackson WB, Davis PL, Groh V, Champlin R. Adenovirus type 19 keratoconjunctivitis in Canada. Can J Ophthalmol. 1975;10(3):326-33. 9. Laibson PR, Ortolan G, Dupre-Strachan S. Community and hospital outbreak of epidemic keratoconjunctivitis. Arch Ophthalmol. 1968;80(4):467-73. 10. Stasiuk RM, Robertson IF. Adeno-viral kerato conjunctivitis. Aust J Ophthalmol. 1981;9(1):55-8. 11. Faden H, Wynn RJ, Campagna L, Ryan RM. Outbreak of adenovirus type 30 in a neonatal intensive care unit. J Pediatr. 2005;146(4):523-7. 12. Viney KA, Kehoe PJ, Doyle B, et al. An outbreak of epidemic keratoconjunctivitis in a regional ophthalmology clinic in New South Wales. Epidemiology and Infection. 2008;136(9):1197-206. 13. Warren D, Nelson KE, Farrar JA, et al. A large outbreak of epidemic keratoconjunctivitis: Problems in controlling nosocomial spread. J Infect Dis. 1989;160(6):938-43. 14. Udeh BL, Schneider JE, Ohsfeldt RL. Cost effectiveness of a point-of-care test for adenoviral conjunctivitis. The Am J of the Med Sciences. 2008;336(3):254-64. 15. Kuo IC, Espinosa C, Forman M, Valsamakis A. A polymerase chain reaction-based algorithm to detect and prevent transmission of adenoviral conjunctivitis in hospital employees. Am J Ophthalmol. 2016;163:38-44. 16. Meyer-Rusenberg B, Loderstadt U, Richard G, Kaulfers PM, Gesser C. Epidemic keratoconjunctivitis: The current situation and recommendations for prevention and treatment. Review. Dtsch Arztebl Int. 2011;108(27):475-80. 17. Kaufman HE. Adenovirus advances: New diagnostic and therapeutic options. Curr Opin Ophthalmol. 2011;22(4):290-3. 18. Ghebremedhin B. Human adenovirus: Viral pathogen with increasing importance. Eur J Microbiol Immunol (Bp). 2014;4(1):26-33. 19. Aoki K, Tagawa Y. A twenty-one year surveillance of adenoviral conjunctivitis in Sapporo, Japan. Int Ophthalmol Clin. 2002;42(1):49-54. 20. Than T, Morettin CE, Harthan JS, et al. Efficacy of a single administration of 5% povidone-iodine in the treatment of adenoviral conjunctivitis. Am J Ophthalmol. 2021;231:28-38. 21. Liu SH, Hawkins BS, Ng SM, et al. Topical pharmacologic interventions versus placebo for epidemic keratoconjunctivitis. Cochrane Database Syst Rev. 2022;3(3):CD013520. 22. Kaufman HE. Adenovirus advances: new diagnostic and therapeutic options. Curr Opin Ophthalmol. 2011;22(4):290-3. 23. Ward JB, Siojo LG, Waller SG. A prospective, masked clinical trial of trifluridine, dexamethasone, and artificial tears in the treatment of epidemic keratoconjunctivitis. Cornea. 1993;12(3):216-21. 24. Roba LA, Kowalski RP, Gordon AT, et al. Adenoviral ocular isolates demonstrate serotype-dependent differences in in vitro infectivity titers and clinical course. Cornea. 1995;14(4):388-93. 25. Gigliotti F, Williams WT, Hayden FG, et al. Etiology of acute conjunctivitis in children. J Pediatr. 1981;98(4):531-36. 26. Rietveld RP, van Weert HC, ter Riet G, et al. Diagnostic impact of signs and symptoms in acute infectious conjunctivitis: systematic literature search. BMJ. 2003; 327:789. 27. Matsui K, Shimizu H, Yoshida A, Nagaoka E, Nishio O, Okuda K. Monitoring of adenovirus from conjunctival scrapings in Japan during 2005-2006. J Med Virol. 2008;80(6):997-1003. 28. Akcay E, Carhan A, Hondur G, et al. Molecular identification of viral agents associated with acute conjunctivitis: A prospective controlled study. Braz J Infect Dis. 2017;21(4):391-5. 29. Ishii K, Nakazono N, Fujinaga K, et al. Comparative studies on etiology and epidemiology of viral conjunctivitis in three countries of East Asia: Japan, Taiwan and South Korea. Int J Epidemiol. 1987;16(1):98-103. 30. Torres Rojas G, Goyenechea A, Savon C, Valdes O, Oropesa I. The incidence of adenoviruses in viral conjunctivitis. Rev Cubana Med Trop. 1998;50(3):182-5. 31. Morris DJ, Bailey AS, Cooper RJ, et al. Polymerase chain reaction for rapid detection of ocular adenovirus infection. J Med Virol. 1995;46(2):126-32. 32. Lee CS, Lee AY, Akileswaran L, et al. Determinants of outcomes of adenoviral keratoconjunctivitis. Ophthalmol. 2018;125:1344-53. 33. Sambursky R, Trattler W, Tauber S, et al. Sensitivity and specificity of the AdenoPlus test for diagnosing adenoviral conjunctivitis. JAMA Ophthalmol. 2013;131:17-22. 34. Kam KY, Ong HS, Bunce C, et al. Sensitivity and specificity of the AdenoPlus point-of-care system in detecting adenovirus in conjunctivitis patients at an ophthalmic emergency department: A diagnostic accuracy study. Br J Ophthalmol. 2015;99:1186-89. 35. Holtz KK, Townsend KR, Furst JW, et al. An assessment of the AdenoPlus point-of-care test for diagnosing adenoviral conjunctivitis and its effect on antibiotic stewardship. Mayo Clin Proc Innov Qual Outcomes. 2017;1:170-75. 36. Johnson SD, Harthan JS, Than T, et al. Predictive accuracy and densitometric analysis of point-of-care immunoassay for adenoviral conjunctivitis. Transl Vis Sci Technol. 2021;10:30. 37. Than T, Hartwick A, Shorter E, et al. How to manage adenoviral conjunctivitis. Optometry Times. Published February 8, 2014. www.optometrytimes.com/view/how-manage-adenoviral-conjunctivitis. Accessed October 15, 2022. 38. Shekhawat NS, Shtein RM, Blachley TS, Stein JD. Antibiotic prescription fills for 392 acute conjunctivitis among enrollees in a large United States managed care 393 network. Ophthalmol. 2017;124(8):1099-107. 39. Optometric Clinical Practice Guideline. Care of the patient with conjunctivitis. American Optometric Association. November 8, 2002. 40. Preferred Practice Pattern Guidelines. Conjunctivitis- limited revision. American Academy of Ophthalmology Cornea/External Disease Panel. 2018. 41. Abel AD. Adenoviral conjunctivitis. How to use povidone-iodine for treatment. Ophthalmology Management. Published February 2000. www.ophthalmologymanagement.com/issues/2000/february-2000/adenoviral-conjunctivitis. Accessed October 15, 2022. 42. Abelson M, Shapiro A. A guide to understanding adenovirus, the diseases it causes and the best ways to treat these conditions. Rev Ophthalmol. Published 2010. www.reviewofophthalmology.com/article/a-guide-to-understanding-adenovirus-the-diseases-it-causes-and-the-best-ways-to-treat-these-conditions. Accessed October 15, 2022. 43. Shovlin JP. What’s the buzz about betadine? Review of Ophthalmol. Published September 15, 2011. www.reviewofoptometry.com/article/whats-the-buzz-about-betadine. Accessed October 15, 2022. 44. Thomas R, Melton R, Vollmer P. Clinical perspectives in patient care 25th edition. Rev Optom Supp. 2021. 45. Pernicky J. Personal experience with the use of Betadine gtt. in the treatment of epidemic viral keratoconjunctivitis. Cesk Oftalmol. 1994;50(5):323-4. 46. Kuo IC, Gower EW. Cost savings from a policy to diagnose and prevent transmission of adenoviral conjunctivitis in employees of a large academic medical center. JAMA Ophthalmol. 2021;139:518-24. 47. Lee SK, Zhai H, Maibach HI. Allergic contact dermatitis from iodine preparations: A conundrum. Contact Dermatitis. 2005;52:184-7. 48. Lachapelle JM. A comparison of the irritant and allergenic properties of antiseptics. Eur J Dermatol. 2014;24(1):3-9. 49. Peden MC, Hammer ME, Suner IJ. Dilute povidone-iodine prophylaxis maintains safety while improving patient comfort after intravitreal injections. Retina. 2019;39(11):2219-24. 50. Marks JG. Allergic contact dermatitis to povidone-iodine. J Am Acad Dermatol. 1982;6:473-5. 51. Whiteside M, Shorter E, Migneco M, et al. Reducing Adenoviral Patient Infected Days (RAPID) study: Success in masking subjects and clinicians from identifying treatment with ophthalmic povidone-iodine 5% (PVP-I). Invest Ophthalmol Vis Sci. 2018;59(9). 52. Butt AL, Chodosh J. Adenoviral keratoconjunctivitis in a tertiary care eye clinic. Cornea. 2006;25(2):199-202. 53. Shorter EL, Whiteside MM, Harthan JS, et al. Diagnostic accuracy of clinical signs, symptoms and point-of-care testing for early adenoviral conjunctivitis. Clin Exp Optom. 2022;105:7:702-7. |