Arsenic, a naturally occurring element in the earth’s crust, is widely distributed throughout the environment. It has been used as a medicinal agent, pesticide and herbicide, in metallurgical applications, and in the manufacturing of certain types of glass, microchips, and paint pigments.1-4 Arsenic exists in two forms: organic and inorganic. The inorganic form is known to be more toxic and diagnostic of arsenic poisoning when elevated levels are found in the bloodstream. In the United States, arsenic exposure is usually secondary to occupational exposure, contaminated wine or moonshine, or suicidal or homicidal intent.5 There is also the possibility of arsenic contamination in nutritional and herbal preparations.
Some countries, such as Bangladesh and West Bengal, have increased arsenic levels in the drinking water due to arsenic-containing rocks near the water supply.7,8 Once toxic levels are reached, essentially every organ in the body is affected, including the heart, blood/bone marrow, liver and skin.9,10 Toxic levels lead to impairment of mitochondrial function, which results in optic neuropathy and peripheral neuropathy.7,11-13
While arsenic poisoning is a rare cause of optic neuropathy, it has the potential for devastating consequences, including death. Therefore, it is imperative to consider arsenic poisoning when presented with cases of optic neuropathy.
History
A 40-year-old black male was referred to Ocular Diagnostics and Medical Services for an unexplained visual decrease in both eyes. This patient, an unemployed mason, was from Colombia but had been the in United States for eight years.
His ocular history revealed a gradual loss of vision during the past seven years that had been especially noticeable in the last five years. He said the loss was painless and bilateral. His ocular family history was unremarkable, and he reported that his mother has diabetes.
His systemic history included high cholesterol and upper respiratory infections that recurred approximately every two months. He began taking a multivitamin when he first noticed the vision loss, but was not taking any other medications. The patient denied the use of antimalarials and recreational drugs, and he reported drinking approximately five beers per month; however, he denied the use of any “moonshine.” He also admitted an 8.5-year history of smoking a pack a day.
Diagnostic Data
Upon examination, the patient’s entering uncorrected visual acuity at distance was 20/200 O.U.; there was no improvement upon pinhole testing. Confrontation visual fields were full to finger counting in both eyes. Pupils were equal, round and sluggishly reactive to light. Slit lamp exam revealed nasal and temporal pinguecula on both eyes and a slight nasal pterygium in the right eye. Both corneas were clear.
Intraocular pressures with Goldmann applanation tonometry measured 12mm Hg O.U. at 10:55 a.m. A dilated fundus exam showed cup-to-disc ratios of 0.30 x 0.30 O.D. and 0.40 x 0.40 O.S., with temporal pallor and optic atrophy in both eyes. A pigmented chorioretinal scar measuring approximately one disc diameter in size was documented in the superotemporal periphery of the right eye.
The maculae of both eyes exhibited slight retinal pigment epithelial changes. Humphrey visual field testing with a 30-2 SITA standard strategy revealed cecocentral scotomas in both eyes. Subsequently, 30-2 short-wave automated perimetry (SWAP) was performed and did not differ significantly from standard achromatic perimetry. Lastly, fundus photos were taken of the nerve (figures 1 and 2).
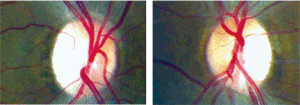 |
| 1, 2. Fundus photos of the optic nerves (O.D. left, O.S. right). |
Tentative Diagnosis and Follow-Up
The tentative assessment at this visit was optic atrophy of an unknown etiology. We instructed the patient to return to the clinic in one week for Farnsworth D-15 test, optical coherence tomography, a GDx scan, an electroretinogram (ERG), visual-evoked potential (VEP) test and blood testing.
Further Diagnostic Data
One week later, Farnsworth D-15 saturated and unsaturated testing revealed a tritan defect in both eyes. A GDx scan was performed, but results were not reliable due to poor central fixation. (Studies have shown, however, that scanning laser polarimetry has poor sensitivity to detect axonal loss in the nasal and temporal areas of the optic disc.14)
An ERG was performed and indicated normal rod and cone function, as well as normal inner and outer retinal layer response in both eyes. The VEP showed reduced amplitudes corresponding to the decreased visual acuity, but implicit time was normal.
At this time, the clinical staff decided to refer the patient to the Good Neighbor Clinic for blood testing. Blood tests ordered include B12, folate, VDRL (venereal disease research lab test for active syphilis), ANA (Antinuclear Antibody Test), FTA-ABS (fluorescent treponemal antibody absorption for latent syphilis) and a urine test for heavy metals.
Approximately three months later, the patient returned to the clinic to learn the results of the blood tests. But, they were non-diagnostic, and the heavy metal screening had not been performed. At this visit, a macular OCT was performed; both maculae were normal.
So, we again referred the patient for further testing, this time to the San Jose Clinic. These were performed two weeks later, and results revealed elevated total arsenic levels in his urine: 125.3µg/l. The arsenic levels were differentiated into organic and inorganic moieties, and the inorganic arsenic levels were high at 36.8µg/l. (Total arsenic levels should not exceed 35µg/l, and the inorganic arsenic levels should not exceed 5µg/l.)
The patient denied any exposure to heavy metals at work or home; the vector of toxicity was unknown. The patient reported no signs of peripheral neuropathy at this time, but hyperkeratosis and hyperpigmentosis were noted on his back and the soles of his feet (figures 3 and 4). A PPD (purified protein derivative) skin test revealed active tuberculosis with an induration of 24mm in diameter. The patient had never been diagnosed with tuberculosis.
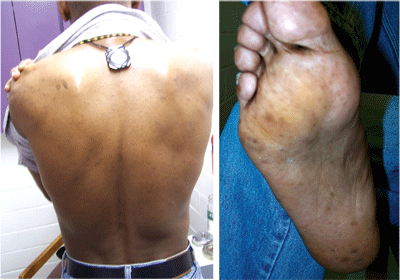 |
| 3, 4. Hyperkeratosis and hyperpigmentosis, both signs of arsenic poisoning, were noted on the patient’s back and on the soles of his feet. |
We diagnosed the patient with arsenic poisoning and tuberculosis.
Follow-Up and Management
We referred the patient to a neurologist for treatment of arsenic poisoning, and to an internist for treatment of tuberculosis. Sadly, the patient was lost to follow-up despite repeated phone calls and letters. His phone was disconnected shortly after he stopped answering it. I contacted the city police department and the district attorney’s office, but I was informed that they would only investigate the case if I knew for sure a specific person was poisoning him. I was suspicious of his wife’s behavior, but there was not sufficient evidence to investigate.
Discussion
Arsenic is a highly toxic metalloid that originates from man-made and natural sources. Arsenic is used to protect wood from dry rot, fungi, molds, termites, and other pests.15 Arsenic is used in metallurgy for hardening copper, lead and other alloys. It aids in manufacturing certain types of glass and microchips, paints and fungicides and pesticides.2-4 Arsenic is released during the smelting of copper ores, mining, pulp and paper production, cement manufacturing, and in the burning of fossil fuels and waste products.
Sources of natural arsenic include volcanism, forest fires and ground water. Currently in the U.S., arsenic poisoning is most likely due to industrial exposure, contaminated alcohol or malicious intent.5 There is also the possibility of contamination of nutritional supplements and herbal preparations, such as Indian Ayurvedic medicines.16 Our patient denied any possible exposure from any of these sources.
Arsenic is absorbed through the gastrointestinal tract, though minimal absorption may occur through the skin. Once arsenic enters the body, it binds to hemoglobin, plasma, proteins and leukocytes, and it is dispersed through the entire body.9 Over the next few weeks, arsenic can be found in the skin, hair, nails, bone, muscle, and even nervous tissue. Toxicity is more pronounced in the nerves, heart, liver, and blood or bone marrow.9,10
Two mechanisms of arsenic toxicity have been described. Arsenic binds with sulfhydryl groups and disrupts sulfhydryl-containing enzymes.7,11-13 The pyruvate and succinate oxidation pathways are inhibited, as are the tricarboxylic acid cycle, gluconeogenesis and oxidative phosphorylation.7,11-13
A second mechanism involves substitution of arsenic for phosphorus in many biochemical reactions. Replacing the stable phosphorus anion in phosphate with the less stable arsenic anion leads to rapid hydrolysis of high-energy bonds in such compounds as adenosine tri-phosphate, which leads to the loss of high-energy phosphate bonds and effectively uncouples oxydative phosphorylation, impairing mitochondrial function.7,11-13
Mitochondria are the primary cellular source of reactive oxygen species (ROS), which may cause apoptosis and cellular death. The mechanism of mitochondrial dysfunction’s effects on the central nervous system are not understood; however, hypotheses suggest that energy depletion and accumulation of ROS predict the vulnerability of specific axons in the central and peripheral nervous system.7,11-13 Impaired oxidative phosphorylation and increased ROS production may cause the opening of the mitochondrial permeability transition pore, which activates the apoptotic cascade and results in cell death.7,11-13
Mitochondrial dysfunction has a negative impact on axonal transport, which also leads to mitochondrial impairment and energy depletion distally. Those axons that are highly vulnerable to energy depletion and ROS accumulation are small in caliber, long, unmyelinated or poorly myelinated, and have a rapid firing rate. The papillomacular bundle is quickly damaged, resulting in impaired central vision. The incidence of optic atrophy increases with the dosage.7,11-13 The peripheral nervous system is susceptible and results in peripheral neuropathy. Arsenic may also enhance susceptibility to infections by disrupting glucocorticoids, which help regulate the immune system.17
Who is Susceptible?
Arsenic poisoning can affect anyone of any race or age. Children may encounter arsenic as a pesticide, whereas adults are more likely to be exposed through industry or occupational environments. Arsenic toxicity is more prevalent in areas with elevated amounts of arsenic in the water supply, such as Bangladesh and West Bengal.7,8
Most countries throughout the world have arsenic levels of less than 50ppb, which is thought safe; however, chronic exposure leads to an increased risk of cancer.8 As a result, the U.S. has recently established a new Maximum Contaminant Level for arsenic: 10ppb in drinking water.
A careful, complete patient history is the key to revealing arsenic exposure. Total arsenic levels in the urine should not exceed 35µg/l. Additionally, inorganic arsenic levels should not be more than 5µg/l. The acute lethal dose of inorganic arsenic has been estimated at 0.6mg/kg/day.15 This indicates that for a 150lb adult, a toxic dose is 42mg; for a 20lb child, the toxic dose is 6mg.
A patient with chronic arsenic poisoning may present with fatigue, weight loss, gastrointestinal disturbances and respiratory irritation, as well as slowly progressive central vision loss and dyschromatopsia. Vision loss is usually bilateral, symmetric and painless. Acuity may vary from minimal reduction to no light perception, but patients usually present with 20/200 or better.
Early symptoms of peripheral nerve damage, such as pain, tingling and numbness in the extremities, have also been reported.5 Patients with arsenic poisoning will usually have a garlic-like smell to their breath and tissue fluids. Upon examination, hair loss, hypertension, ulceration of the nasal septum, dermatitis and anemia are often noted. Hyperpigmentation and hyperkeratosis of the skin—particularly on the palms and soles of the feet—is common.5,7 “Black foot disease” (gangrene of the feet), renal damage and cirrhosis of the liver have also been reported.5,7
Long-term exposure can cause the development of white, crescent-moon marks on the fingernails, known as Mees lines. Optic atrophy may present with a central or cecocentral scotoma, which spares the periphery.18 Eventually, optic disc pallor results, signaling loss of the papillomacular bundle.18 Associated swelling of the nerve fiber layer in the arcuate bundles above and below the papillomacular bundle may also be seen. Arsenic is a known carcinogen that can ultimately cause skin cancer as well as internal cancers.7
Diagnostic Options
When presented with an optic atrophy of unknown etiology, blood testing and a heavy metal screening are required. Testing may include ESR(erythrocyte sedimentation rate), FTA-ABS, RPR-VDRL, CBC, serum folate and vitamins B1, B2, and B12, PPD, and a heavy metal urinalysis screening.19
If total arsenic levels are high, the lab must differentiate the arsenic into organic and inorganic moieties. This specification is imperative; relying on total arsenic levels alone could lead to unnecessary treatment for many patients.
There is no specific treatment for toxic optic atrophy, but prompt management may ameliorate or prevent severe visual deficit.20,21 If the cause of toxic optic atrophy can be identified early, removal of the toxin may result in significant recovery over a period of weeks to months.20,21 However, long-standing optic atrophy often results in permanent vision loss.
In the case of arsenic poisoning, the patient should be referred to a neurologist for further treatment and testing. Appropriate treatment must also be given to other underlying systemic effects, as was the case with our patient.
The possibility of arsenic exposure can be easily neglected because it is rarely encountered in the United States. However, it is imperative for optometrists to consider arsenic poisoning when presented with a case of optic neuropathy. Early diagnosis is the key for this condition and offers the best prognosis visually, as well as systemically, for the affected patient.
Dr. Pate is a Clinical Associate Professor at the University of Houston College of Optometry. He is an attending in the Ocular Diagnostic Services. Dr. Hudson practices in Pampa, Tex., at the Panhandle Eye Group.
1. Monteiro MLR, Medeiros FA, Ostroscki MR. Quantitative analysis of axonal loss in band atrophy of the optic nerve using scanning laser polarimetry. Br J Ophthalmol. 2003 Jan;87(1):32-7.
2. Edlich RF, Winters KL, Long WB 3rd. Treated wood preservatives linked to aquatic damage, human illness, and death—a societal problem. J Long Term Eff Med Implants. 2005;15(2):209-23.
3. CCA Wood and Arsenic: Toxicological Effects of Arsenic. Available at: www.origen.net/arsenic.html (Accessed January 2009).
4. Department of Health and Human Services. CDC Fact Sheet: Arsenic and Drinking Water from Private Wells. Available at: www.cdc.gov (Accessed December 2009).
5. Marcus S. Toxicity, Arsenic. Available at: www.emedicine.com (Accessed December 2009).
6. Saper RB, Kales SN, Paquin J, et al. Heavy metal content of ayurvedic herbal medicine products. JAMA. Dec 2004; 292(23):2868-73.
7. Harvard University Study. Chronic Arsenic Poisoning: History, Study, and Remediation. Available at: www.physics.harvard.edu/~wilson/arsenic/arsenic_project_introduction.html (Accessed December 2009).
8. Ng JC, Moore MR. Arsenic in drinking water: a natural killer in Bangladesh and beyond. Med J Australia. 2005;183(11/12):562-3.
9. Graziano C. Toxicity, Arsenic. Available at: www.emedicine.com (Accessed December 2009).
10. Chemical Safety Information from Intergovernmental Organizations. Arsenic. Available at: www.inchem.org (Accessed November 2009).
11. Sadun AA. Metabolic optic neuropathies. Semin Ophthalmol. 2002 Mar; 17(1):29-32.
12. Sadun AA. Mitochondrial optic neuropathies. J Neurol Neurosurg Psychiatry. 2002 Apr;72(4):423-5.
13. Carelli V, Ross-Cisneros FN, Sadun AA. Optic nerve degeneration and mitochondrial dysfunction: genetic and acquired optic neuropathies. Neurochem Int. 2002 May;40(6):573-84.
14. University of California Santa Cruz Lab Safety Services. Arsenic Substance Technical Guidelines. Available at: http://ehs.ucsc.edu (Accessed November 2009).
15. Ratnaike RN. Acute and chronic arsenic toxicity. Postgrad Med J 2003 Jul;79(933):391-6.
16. Van Stavern GP, Newman NJ. Optic neuropathies. An overview. Ophthamol Clin North Am. 2001 Mar;14(1):61-71,viii.
17. University of Otago New Zealand Geology Department. Arsenic in the Environment. Available at: www.otago.ac.nc/geology/features/metals/arsenic.html (Accessed November 2009).
18. Kesler A, Pianka P. Toxic optic neuropathy. Curr Neurol Neurosci Rep. 2003 Sep;3(5):410-4.
19. Onofrey BE, Skorin L, Holdeman NR. Ocular Therapeutics Handbook: A clinical Manual. Second Ed. Philadelphia, PA: Lippincott Williams & Wilkins. 2005.
20. Duker AA, Carranza EJ, Hale M. Arsenic geochemistry and health. Environ Int. 2005 Jul;31(5):631-41.
21. Kalia K, Flora SJS. Strategies for safe and effective therapeutic measures for chronic arsenic and lead poisoning. J Occup Health. 2005 Jan;47(1):1-21.

