Seeing Systemic Disease within the EyeThis month in Review of Optometry, multiple ODs explain the connection between the eye and various systemic conditions, including hypertension, stroke, sleep disorders, COVID-19, diabetes, thyroid eye disease and more. Check out the other articles featured in the October issue:
|
Consider that one-third of the average human’s lifetime is spent sleeping. Sleep is a universal function of living species. Its restorative functions include memory consolidation, hormone regulation, growth and sympathetic/parasympathetic balance.1 Sleep disorders can interfere with normal physical, mental, social and emotional functioning. Insufficient quality or quantity of sleep is associated with system dysfunctions including endocrine, metabolic, higher cortical function and neurological disorders.2,3 Every day, new research helps us learn more about the direct and indirect consequences sleep disorders may have on ocular health.
The biological process of sleep is a reversible state of unconsciousness in which metabolism and motor activity is reduced.4 There are two different kinds of sleep: rapid eye movement (REM) and non-rapid eye movement (NREM).4 NREM typically follows a drowsy state and involves dreamless sleep. It is divided into three stages of sleep: the first (N1) is characterized by a transition from wakefulness to sleep, the second (N2) comprises the largest percentage of total sleep and the third (N3) involves a period of approximately 70 to 80 minutes of deep sleep. Following N3, deep sleep lightens, and REM follows. This part of the cycle is customarily associated with active dreaming and body movements.4
Sleep disorders consist of conditions that are attributed to the disruption of normal sleep patterns.3,4 Although sleep disorders are more common in adults, they can occur during childhood.3 Considered to be most prevalent during childhood, parasomnias are unusual behaviors that one may experience during the course of sleep. Included are sleepwalking, confusional arousals, sleep terrors, sleep talking and nightmares.3,4 Parasomnias are generally not associated with complaints of insomnia or sleepiness but can be associated with possible injury (due to irregular movements and mobility) and negative psychosocial effects.5
Disorders of sleep are associated with complaints of insufficient sleep, altered amounts of perceived sleep or abnormal movements during sleep.2 The physical examination of a patient suspected of having a sleep disorder may include signs of poor concentration, reported drowsiness, slowed reaction time, hypertension (HTN), enlarged tonsils and a narrowed airway. The major causes of sleep disorders are medical and psychological conditions.4
 |
|
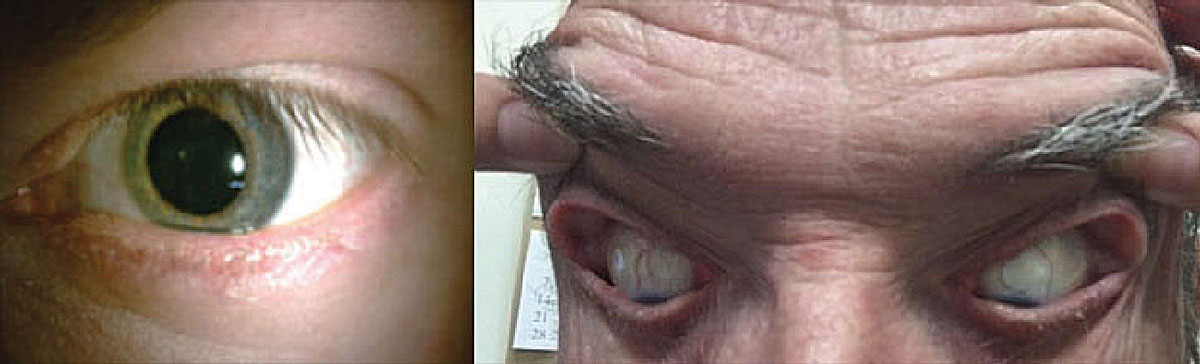
Patients with OSA may develop lash ptosis (left) and/or floppy eyelid syndrome (right). Photo: Victoria Roan, OD. Click image to enlarge. |
Medical Conditions and Sleep Disorders
There are many medical conditions that have been associated with sleep disorders. Cardiovascular disease may cause the patient to wake during sleep due to the feeling of shortness of breath.2-4 Neurologic diagnoses including stroke, hypnic jerk (sudden, involuntary muscle contractions that occur as you are falling asleep) and restless leg syndrome (RLS) are associated with unwanted movements that disrupt sleep.2-4,6-9 Other neurologic diagnoses that cause sleep disruption include central sleep apnea, headache and central degenerative disorders such as Alzheimer’s disease, Parkinson’s disease, dementia and amyotrophic lateral sclerosis.4,10
Endocrine disorders, including metabolic syndromes, may disrupt the sleep-wake cycle as hormone regulation is altered. Hyperthyroidism, diabetes mellitus and vitamin D deficiency are among these common disorders.2-4 Pregnancy and menopause also affect hormone regulation and may impact sleep.
Chronic obstructive pulmonary disease and asthma are common pulmonary diseases that affect sleep due to difficulty breathing or compromised breathing patterns. Obstructive sleep apnea (OSA) is a pulmonary disorder associated with the collapse of the upper airway during sleep.2-4,11
The gastrointestinal diagnosis, gastroesophageal reflux disease, is often associated with the disruption of sleep due to the flareup of symptoms when a patient lays down to go to sleep.2-4 When laying down, gravity no longer assists in keeping stomach acids contained, causing reflux into the esophagus. Symptoms of heart burn and chest pain can develop and awaken patients from sleep.4
Musculoskeletal conditions causing acute or chronic pain, such as an acute injury, arthritis and fibromyalgia, cause patients to wake more during the course of sleep resulting in reduced quality and quantity of sleep. Patients who live with pain learn how to position themselves when sleeping to avoid uncomfortable posture.
Psychological Conditions and Sleep Disorders
Sleep disorders are common among patients who suffer from depression and result in both insomnia (trouble falling and/or staying asleep) and hypersomnia (episodes of excessive daytime sleepiness or prolonged nighttime sleep).2,3 Anxiety also predisposes patients to insomnia as they may have trouble falling asleep, staying asleep and feeling rested upon awakening. Panic attacks may occur during both light and deep sleep, resulting in sleep disruption.4
Medications used to treat psychological conditions may cause patients to have difficulty falling asleep and have an impact on sleep patterns.3 Antidepressant medications can disrupt REM sleep patterns. Most commonly associated with treatment in elderly patients, benzodiazepines can cause rebound insomnia if a dosage change is made or a medication is discontinued.4
Classification of Sleeping Disorders
 |
|
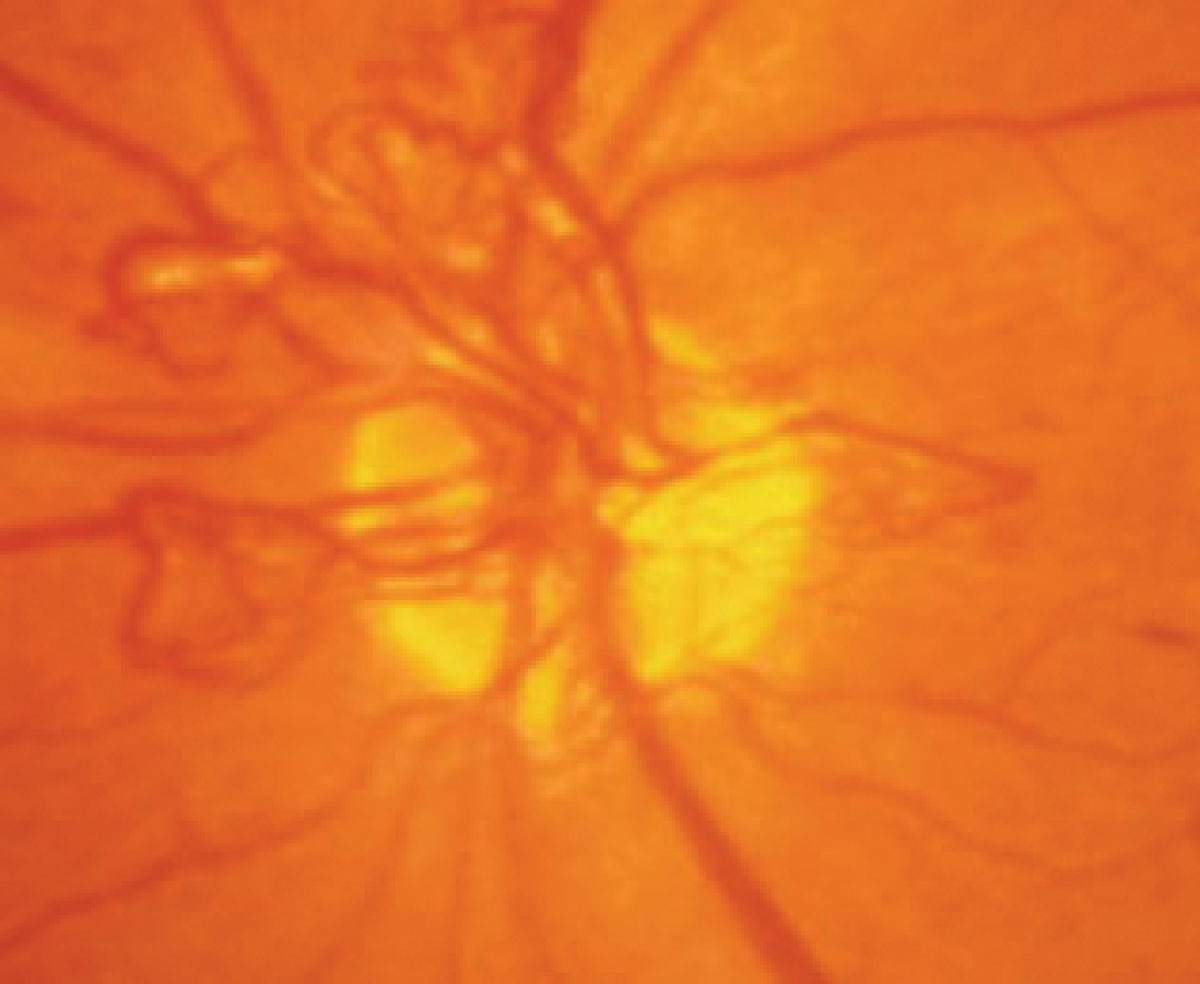
Sleep apnea has been linked to diabetes-related ocular conditions. Photo: Paul Chous, OD. Click image to enlarge. |
Sleep disorders that occur in adults manifest as complaints of insufficient sleep, excessive perceived sleep or abnormal movements during sleep.1,5,8 Transient insomnia is very common and may present in one-third of adults. In many instances, insomnia becomes a more chronic and persistent problem if behavioral, emotional, cognitive and/or medical issues are also present.2-4
There is a belief that sleep is primarily controlled by two regulatory mechanisms: a circadian mechanism that determines the timing of sleep and wakefulness and a homeostatic mechanism that determines the need for sleep and sleep intensity.6 The homeostatic mechanism is thought to determine the need for sleep, or sleep propensity, based on prior wake time, or how long someone has gone without sleep. Circadian rhythm sleep disorders are influenced by homeostatic factors as well as the circadian system.2,4,6
The circadian rhythm in humans is thought to be an endogenously generated system that lasts approximately 24 hours in duration.12-14 The suprachiasmatic nucleus (SCN) is located in the anterior part of the hypothalamus and is considered the pacemaker of the circadian timing system. Efferent projections from the SCN innervate structures including the pineal gland, producing melatonin. Normally, the sleep phase of the circadian rhythm occurs about two hours after the onset of melatonin secretion.12-14 Sleep-wake phase disorders may occur when society-driven sleep times delay (sleep occurs later than needed) or advance (sleep occurs sooner than needed) the sleep phase of the circadian cycle.
Sleep-related breathing disorders are associated with insufficient patterns of sleep.2-4,11,15 There are three types of sleep apnea: obstructive, central and complex. OSA occurs when there is a cessation of air flow for at least 10 seconds and is the result of the collapse of the upper airway during sleep. The prevalence of diagnosed OSA has been increasing in the United States due to increasing rates of obesity and overall awareness of the disorder.11,15
Central sleep apnea is the interruption of air flow that occurs due to a lack of effort to breathe, most commonly originating from the brain respiratory centers and flowing to the muscles that control breathing. Complex sleep apnea occurs as a result of the combination of both obstructive and central apnea.11,15
Sleep-related movement disorders include RLS and periodic limb movement disorder (PLMD). RLS, also known as Willis-Ekbom disease, is described as an irresistible restlessness and urge to move the legs, and less commonly the arms and trunk, due to painful and uncomfortable sensations.7,8 The principal abnormality in RLS is identified as a dopamine deficiency and dysregulation of iron by the brain. RLS is thought to affect 5% to 8% of the general population, with women and adults over 40 more likely to experience the condition.7,8
PLMD is also referred to as sleep-related myoclonus syndrome or nocturnal myoclonus syndrome.9 Although the pathogenesis of PLMD is not clearly defined, dopamine deficiency could be an underlying factor that triggers the spinal flexor pathways resulting in increased limb movements during sleep. Patients commonly complain of disturbed sleep due to repetitive lower extremity movements in the form of extension of the big toe and flexion of the ankle, knee and hip.9 These movements may be associated with autonomic arousal resulting in a change in heart rate and blood pressure or cortical arousal causing frequent nocturnal awakenings. Patient-reported symptoms also include complaints of non-restorative sleep and daytime sleepiness and fatigue affecting their overall livelihood.2,9
 |
|
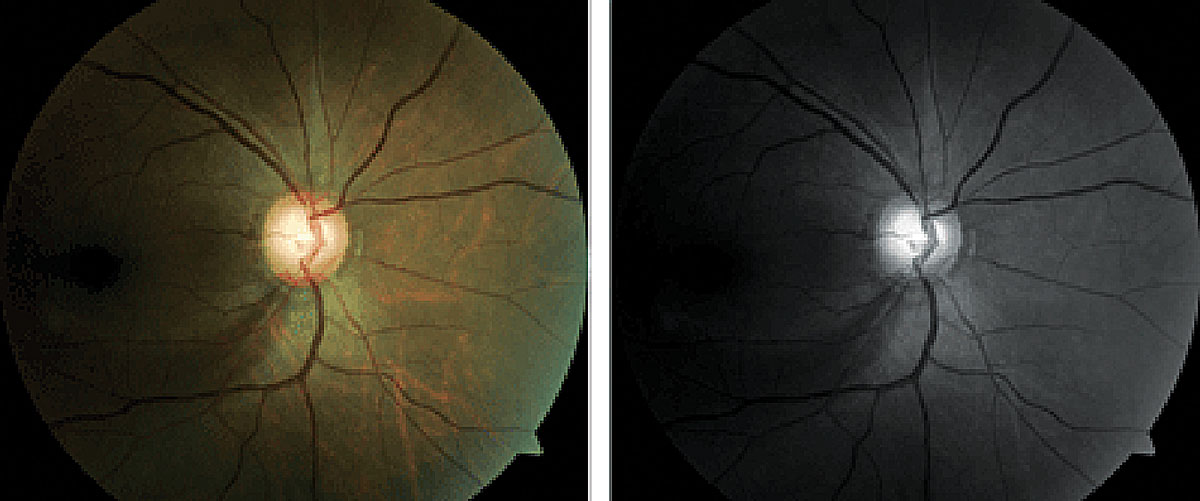
NTG has been a fairly common report in sleep apnea. Photo: Brad Dutton, OD. Click image to enlarge. |
Ocular Sequelae of of Sleeping Disorders
Sleep disorders and their treatment may directly and indirectly compromise ocular health.2-4 The pathophysiologic mechanisms of sleep disorders are thought to influence extracerebral physiologic functions that when compromised result in cardiovascular disease, cerebrovascular disease and metabolic imbalances (increasing the risk of obesity and diabetes).16 In addition, lifestyle modifications, pharmacologic therapies and therapeutic modalities such as continuous positive airway pressure (CPAP) are associated with ocular disease.16
Prolonged eyelid closure during sleep serves as a mechanical barrier between the ocular surface and the external environment.16,17 It also reduces tear production. REM promotes the transfer of aqueous humor, increasing nutrient supply to the cornea and compensating for the reduction in tear production and oxygen to the cornea. Frequent nocturnal awakenings due to a sleep disorder may result in a patient developing signs and symptoms of dry eye as the result of a compromised ocular surface.16,17
OSA is an independent risk factor for the development of HTN and cardiovascular and cerebrovascular diseases such as obesity, diabetes, ischemic heart disease and psoriasis. Considering the consequences these systemic diseases have on the vascular system, it is no surprise that several ophthalmologic diseases are linked to OSA.2,3,11 These diagnoses include floppy eyelid syndrome, primary open-angle glaucoma (POAG), normal-tension glaucoma (NTG), nonarteritic anterior ischemic optic neuropathy (NAION), papilledema and dry eye, as well as CPAP-associated eye complications.2,11,16-18
The signs of floppy eyelid syndrome include easily everted floppy eyelids, eyelash ptosis and papillary conjunctivitis due to spontaneous eyelid eversion. Patients may present with symptoms that include watering, stickiness, discomfort and blurred vision that is usually bilateral and most oftentimes worse upon awakening in the morning.16-19 Clinical presentation may include ptosis, misdirected (most often downward) or inverted eyelashes, papillary conjunctivitis and corneal involvement. Corneal findings can include punctate keratopathy, epithelial defect, surface scarring, neovascularization, keratoconus and infectious keratitis, among others.16-19
The exact pathophysiology of floppy eyelid syndrome is not completely understood. Studies have suggested the role of mechanical stress, alternating periods of ischemia and reperfusion causing tissue inflammation and loss of elastin fibers in the tarsal plate. An increase in the immunoreactivity of the eyelid elastolytic processes, in particular an increase in matrix metalloproteinase, likely accounts for the loss of elastin fibers.19
CPAP-associated eye complications are thought to arise from two possible mechanisms. Most commonly, air leaks around the superior portion of the mask causing air to blow onto the ocular surface. The other mechanism involves retrograde movement of air and mucus from the nasal passage through the nasolacrimal duct and onto the ocular surface. Consequences of these mechanisms include dry eye, increased mucoid discharge on the ocular surface, blepharitis and episodes of bacterial conjunctivitis.11,20 It is best to avoid sleeping in an extended-wear contact lens when using CPAP.
In addition to ocular surface complications, patients often present with skin irritation and breakdown due to a poorly fit mask or dry air flow. Most often adjustments made to the fit of the mask and/or to the humidity of air flow solve these skin issues. Switching to a nasal interface may be required to eliminate areas of pressure on the skin or to reduce the flow of air that escapes the superior border of the mask.16
Treatment of ocular complications associated with CPAP use includes nocturnal lubrication with a viscous artificial tear or ointment.2-4,11,16 Additional daytime lubrication may be needed to quell symptoms reported upon awakening. As with complications involving the nasal passages or facial skin, the health of the ocular surface can be improved by adjusting the fit of the mask or increasing the humidity of the flow of air.11,16
POAG and OSA were reported in 1982 to occur in the same family of diagnoses.21 The number and duration of apneic episodes in cases of OSA correlated directly with the severity of POAG. Since initial reports, NTG has also been associated with OSA. The prevalence of OSA and glaucoma ranges from 2% to 27% vs. the expected 2% in the general population.16
Additional direct correlations that have been identified between patients with moderate to severe OSA and glaucoma include increased incidence of visual field defects and decreased retinal nerve fiber layer thickness.16 The proposed physiologic mechanisms linking OSA to glaucomatous optic nerve damage include direct hypoxic injury, disrupted autoregulation of blood flow due to periods of hypoxia and hypercapnia (excessive carbon dioxide in the blood stream due to inadequate respiration) and blood flow disruption due to periods of HTN that occur during apneic episodes.22
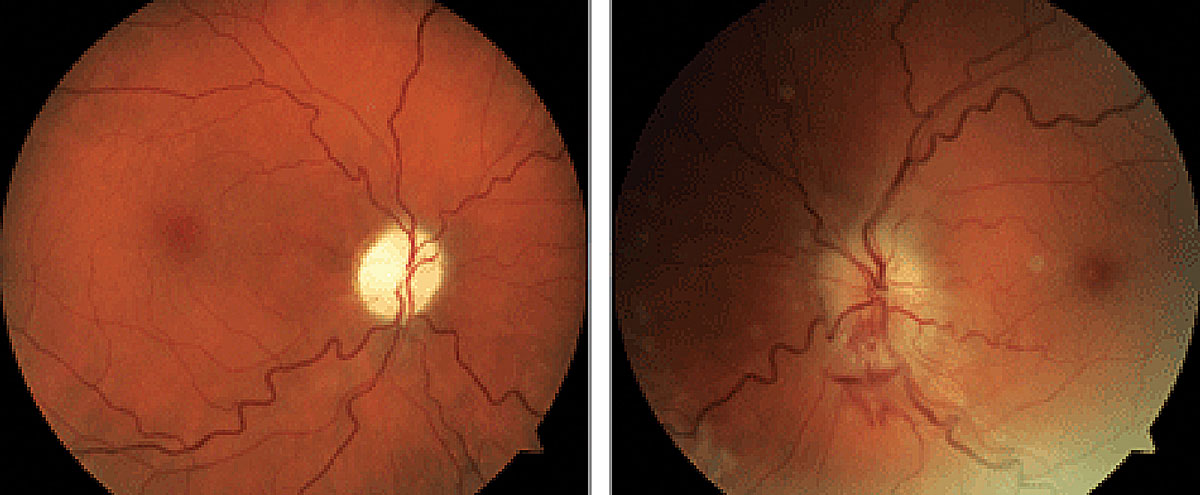 |
NAION and sleep apnea share a significant association. Photo: Brad Sutton, OD. Click image to enlarge. |
Interestingly, POAG has also been associated with circadian rhythm sleep disorders and endogenous melatonin levels. POAG is considered a causal factor for circadian disruption. Glaucoma causes retinal ganglion cell (RGC) damage. Intrinsically photosensitive RGCs (ipRGCs) convey non-image-forming photic information via the retinohypothalamic tract to the SCN.12,18 As ipRGCs are progressively damaged, photic information to the SCN is adversely altered, disrupting circadian rhythms and causing impaired sleep and altered mood. Other neurodegenerative pathologies associated with disruption of circadian rhythms include Alzheimer’s disease, Parkinson’s disease and Huntington’s disease.12,18
Altered circadian rhythms impact endogenous melatonin production, causing deregulation of circadian intraocular pressure (IOP). Melatonin is a hormone produced by the pineal gland in response to darkness, playing a key role in the body’s sleep-wake cycle. Its production is reduced in bright light conditions, and melatonin production decreases with age.16-18 Variability in melatonin levels can obscure circadian IOP rhythms, resulting in less consistent control of IOP and potential progression of glaucoma. The altered “rhythm” of IOP may account for some resistance to IOP-lowering therapy. Studies support the use of endogenously administered melatonin in the management of glaucoma (and in neurodegenerative diseases) to better synchronize circadian rhythms, perhaps improving the efficacy of IOP-lowering therapy. For adults, 0.5mg to 5mg of melatonin taken one hour before bed is considered to be a safe and effective therapy.16-18
Sleep apnea has been documented to be 1.5 to two times more likely to be associated with NAION than HTN or diabetes.2,11,16 Mechanisms believed to explain this associated risk include impaired optic nerve head blood flow autoregulation secondary to the effects of apneic episodes, apnea-induced blood pressure variations or an imbalance between vasodilator (nitric oxide) and vasoconstrictor (endothelin) blood pressure variations. Also, episodic increases in intracranial pressure (ICP) associated with hypercapnia during apneic episodes may impact optic nerve head health as a result of direct compression or impaired circulation.11,16
Care of NAION concentrates on systemic disease management to ultimately reduce the risk of recurrence in the ipsilateral or contralateral eye.16,23 It is recommended to include screening for physical features of OSA in this patient population and to conduct diagnostic testing as well. Sleep study screening (polysomnography) should be performed to identify those patients who would benefit from intervention such as treatment via CPAP and initiate the appropriate therapy accordingly and in a more timely fashion.16,23
Papilledema, bilateral optic disc swelling in the presence of increased intracranial pressure, has been identified as having an association with OSA.16,17,24 A report hypothesized that papilledema in patients with OSA was secondary to transient hypercapnia and the resultant transient increases in ICP. Hence, some patients with OSA may present with normal daytime intracranial pressure. CPAP should be used to treat OSA and may contribute to the effect of acetazolamide in reduced ICP and improved signs and symptoms of papilledema.16,17,24
Takeaways
The improvement in diagnosis and treatment of sleep disorders has been in part due to patient education and public awareness of the prevalence of these disorders.2-4,16-19 Wearable technologies available commercially in the form of wristbands, watches and rings may aid in recognizing and monitoring potentially problematic sleep patterns.
Providers are more aware of early signs and symptoms of sleep disorders and the association with systemic diseases such as HTN, diabetes, obesity and mood disorders. In addition, care has become more efficacious as medical treatments and surgical interventions have improved.2-4,16-19
Over the past several decades, significant gains have been made in understanding the relationship that sleep disorders have with ocular disease.16-20 More timely diagnosis and treatment of these disorders will hopefully occur as the pathophysiologic mechanisms linking these disorders are better understood. Optimally, the coordination of care will flourish between primary care providers, sleep specialists (most frequently pulmonologists) and eyecare providers.
Dr. Myers is an optometrist at the Coatesville Veterans Affairs Medical Center in Coatesville, PA. He lectures in academia and throughout North America and Europe on ocular disease. He has no relevant financial interests to disclose.
1. DelRosso LM, Silvestri R, Ferri R. Restless sleep disorder. Sleep Med Clinic. 2021;16:381-7. 2. Pavlova MK, Latreille V. Sleep disorders. Am J Med. 2019;132(3):392-9. 3. Holder S, Narula NS. Common sleep disorders in adults: diagnosis and management. Am Fam Phys. 2022;105(4):397-405. 4. Karna B, Gupta V. Sleep disorder. StatPearls [Internet]. Treasure Island (FL). 2022. 5. Proserpio P, Terzaghi M, Manni R, et al. Drugs used in parasomnia. Sleep Med Clinic. 2020;15:289-300. 6. McDermott M, Brown D, Chervin RD. Sleep disorders and the risk of stroke. Expert Rev Neurother. 2018;18(7):523-31. 7. Bollu PC, Yelum A, Thakkar MM. Sleep medicine: restless leg syndrome. Missouri Medicine. 2018;115(4):380-7. 8. Gossard TP, Trotti LM, Videnovic A, et al. Restless legs syndrome: contemporary diagnosis and treatment. Neurotherapeutics. 2012;18:140-55. 9. Joseph V, Nagalli S. Periodic limb movement disorder. StatPearls [Internet]. Treasure Island (FL). 2022. 10. Jee HJ, Shin W, Jung HJ, et al. Impact of sleep disorder as a risk factor for dementia in men and women. Biomol Ther. 2020;28(1):58-73. 11. Rundo JV. Obstructive sleep apnea basics. Cleveland Clinic Journal of Med. 2019;86(sup1):2-9. 12. Ma MA, Morrison EH. Neuroanatomy, nucleus suprachiasmic. StatPearls [Internet]. Treasure Island (FL). 2022. 13. Kim MJ, Lee JH, Duffy JF. Circadian rhythm sleep disorders. J Clin Outcomes Manag. 2013;20(11):513-28. 14. Frank MG. Challenging sleep homeostasis. Neurobiol Sleep Circadian Rhythms. 2021;10(1):1-3. 15. Huon LK, Liu SYC, Camacho M, et al. The association between ophthalmologic diseases and obstructive sleep apnea: a systemic review and meta-analysis. Sleep Breath. 2016;20:1145-54. 16. Waller EA, Bendel RE, Kaplan J. Sleep disorders and the eye. Mayo Clin Proc. 2008;83(11):1251-61. 17. Lee SSY, Nilagiri VK, Mackey DA. Sleep and eye disease: a review. Clin Experiment Ophthalmol. 2022;50(3):334-44. 18. Gubin D, Weinert D. Melatonin, circadian rhythms and glaucoma: current perspective. Neural Regeneration Research. 2022;17(8):1759-80. 19. Mojon DS, Goldblum D, Fleischhauer J, et al. Eyelid, conjunctival and corneal findings in sleep apnea syndrome. Ophthalmology. 1999;106(6):1182-5. 20. McNab AA. Floppy eyelid syndrome and obstructive sleep apnea. Ophthal Plast Reconstr Syrg. 1997;13(2):98-114. 21. Walsh JT, Montplaisir J. Familial glaucoma with sleep apnea: a new syndrome? Thorax. 1982;37(11):845-9. 22. Gerghel D, Hosking SL, Orgul S. Autonomic nervous system, circadian rhythms, and primary open angle glaucoma. Surv Ophthalmol. 2004;49(5):491-508. 23. Mojon DS, Mathis J, Zulauf M, et al. Optic neuropathy associated with sleep apnea syndrome. Ophthalmology. 1998;105(5):874-7. 24. Sugita Y, Iijima S, Teshima Y, et al. Marked episodic elevation of cerebrospinal fluid pressure during nocturnal sleep in patients with sleep apnea hypersomnia syndrome. Electroencephalogy Clin Neurophysiol. 1985;60(3):214-9. |